Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts
- PMID: 23502354
- PMCID: PMC3766259
- DOI: 10.1097/MIB.0b013e3182813297
Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts
Abstract
Background: Crohn's disease is characterized by repeated cycles of inflammation and mucosal healing which ultimately progress to intestinal fibrosis. This inexorable progression toward fibrosis suggests that fibrosis becomes inflammation-independent and auto-propagative. We hypothesized that matrix stiffness regulates this auto-propagation of intestinal fibrosis.
Methods: The stiffness of fresh ex vivo samples from normal human small intestine, Crohn's disease strictures, and the unaffected margin were measured with a microelastometer. Normal human colonic fibroblasts were cultured on physiologically normal or pathologically stiff matrices corresponding to the physiological stiffness of normal or fibrotic bowel. Cellular response was assayed for changes in cell morphology, α-smooth muscle actin staining, and gene expression.
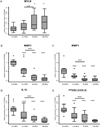
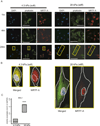
Results: Microelastometer measurements revealed a significant increase in colonic tissue stiffness between normal human colon and Crohn's strictures and between the stricture and adjacent tissue margin. In Ccd-18co cells grown on stiff matrices corresponding to Crohn's strictures, cellular proliferation increased. Pathologic stiffness induced a marked change in cell morphology and increased α-smooth muscle actin protein expression. Growth on a stiff matrix induced fibrogenic gene expression, decreased matrix metalloproteinase, and proinflammatory gene expression and was associated with nuclear localization of the transcriptional cofactor MRTF-A.
Conclusions: Matrix stiffness, representative of the pathologic stiffness of Crohn's strictures, activates human colonic fibroblasts to a fibrogenic phenotype. Matrix stiffness affects multiple pathways, suggesting that the mechanical properties of the cellular environment are critical to fibroblast function and may contribute to auto-propagation of intestinal fibrosis in the absence of inflammation, thereby contributing to the intractable intestinal fibrosis characteristic of Crohn's disease.
Figures






References
-
- Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281. vii. - PubMed
-
- Sands BE, Arsenault JE, Rosen MJ, et al. Risk of early surgery for Crohn's disease: implications for early treatment strategies. Am J Gastroenterol. 2003;98:2712–2718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
