PAX2 Expression in Ovarian Cancer
- PMID: 23502471
- PMCID: PMC3634442
- DOI: 10.3390/ijms14036090
PAX2 Expression in Ovarian Cancer
Abstract
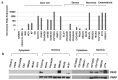
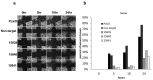
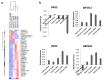
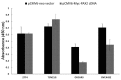
PAX2 is one of nine PAX genes that regulate tissue development and cellular differentiation in embryos. However, the functional role of PAX2 in ovarian cancer is not known. Twenty-six ovarian cancer cell lines with different histology origins were screened for PAX2 expression. Two ovarian cancer cell lines: RMUGL (mucinous) and TOV21G (clear cell), with high PAX2 expression were chosen for further study. Knockdown PAX2 expression in these cell lines was achieved by lentiviral shRNAs targeting the PAX2 gene. PAX2 stable knockdown cells were characterized for cell proliferation, migration, apoptosis, protein profiles, and gene expression profiles. The result indicated that these stable PAX2 knockdown cells had reduced cell proliferation and migration. Microarray analysis indicated that several genes involved in growth inhibition and motility, such as G0S2, GREM1, and WFDC1, were up-regulated in PAX2 knockdown cells. On the other hand, over-expressing PAX2 in PAX2-negative ovarian cell lines suppressed their cell proliferation. In summary, PAX2 could have both oncogenic and tumor suppression functions, which might depend on the genetic content of the ovarian cancer cells. Further investigation of PAX2 in tumor suppression and mortality is warranty.
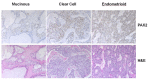
Figures


Similar articles
-
[Significance and expression of PAX8, PAX2, p53 and RAS in ovary and fallopian tubes to origin of ovarian high grade serous carcinoma].Zhonghua Fu Chan Ke Za Zhi. 2017 Oct 25;52(10):687-696. doi: 10.3760/cma.j.issn.0529-567X.2017.10.008. Zhonghua Fu Chan Ke Za Zhi. 2017. PMID: 29060967 Chinese.
-
PAX2, PAX8 and CDX2 Expression in Metastatic Mucinous, Primary Ovarian Mucinous and Seromucinous Tumors and Review of the Literature.Pathol Oncol Res. 2016 Jul;22(3):593-9. doi: 10.1007/s12253-016-0040-2. Epub 2016 Jan 21. Pathol Oncol Res. 2016. PMID: 26797858
-
Overexpression of microRNA-497 suppresses cell proliferation and induces apoptosis through targeting paired box 2 in human ovarian cancer.Oncol Rep. 2016 Oct;36(4):2101-7. doi: 10.3892/or.2016.5012. Epub 2016 Aug 11. Oncol Rep. 2016. PMID: 27513319
-
UnPAXing the Divergent Roles of PAX2 and PAX8 in High-Grade Serous Ovarian Cancer.Cancers (Basel). 2018 Aug 8;10(8):262. doi: 10.3390/cancers10080262. Cancers (Basel). 2018. PMID: 30096791 Free PMC article. Review.
-
[PAX gene function during kidney tumorigenesis: a comparative approach].Bull Cancer. 2006 Sep;93(9):875-82. Bull Cancer. 2006. PMID: 16980230 Review. French.
Cited by
-
Spontaneous Transformation of Murine Oviductal Epithelial Cells: A Model System to Investigate the Onset of Fallopian-Derived Tumors.Front Oncol. 2015 Jul 17;5:154. doi: 10.3389/fonc.2015.00154. eCollection 2015. Front Oncol. 2015. PMID: 26236688 Free PMC article.
-
The Transcoelomic Ecosystem and Epithelial Ovarian Cancer Dissemination.Front Endocrinol (Lausanne). 2022 Apr 28;13:886533. doi: 10.3389/fendo.2022.886533. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35574025 Free PMC article. Review.
-
PAX2 promotes epithelial ovarian cancer progression involving fatty acid metabolic reprogramming.Int J Oncol. 2020 Mar;56(3):697-708. doi: 10.3892/ijo.2020.4958. Epub 2020 Jan 10. Int J Oncol. 2020. PMID: 31922217 Free PMC article.
-
Loss of ARID1A expression leads to sensitivity to ROS-inducing agent elesclomol in gynecologic cancer cells.Oncotarget. 2016 Aug 30;7(35):56933-56943. doi: 10.18632/oncotarget.10921. Oncotarget. 2016. PMID: 27486766 Free PMC article.
-
An integrated epigenome and transcriptome analysis identifies PAX2 as a master regulator of drug resistance in high grade pancreatic ductal adenocarcinoma.PLoS One. 2019 Oct 17;14(10):e0223554. doi: 10.1371/journal.pone.0223554. eCollection 2019. PLoS One. 2019. PMID: 31622355 Free PMC article.
References
-
- Eccles M.R., He S., Legge M., Kumar R., Fox J., Zhou C., French M., Tsai R.W. PAX genes in development and disease: The role of PAX2 in urogenital tract development. Int. J. Dev. Biol. 2002;46:535–544. - PubMed
-
- Chan-Ling T., Chu Y., Baxter L., Weible Ii M., Hughes S. In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: Differentiation, proliferation, and apoptosis. Glia. 2009;57:39–53. - PubMed
-
- Wasco M.J., Pu R.T. Comparison of PAX-2, RCC antigen, and antiphosphorylated H2AX antibody (gamma-H2AX) in diagnosing metastatic renal cell carcinoma by fine-needle aspiration. Diagn. Cytopathol. 2008;36:568–573. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

