Combustion derived ultrafine particles induce cytochrome P-450 expression in specific lung compartments in the developing neonatal and adult rat
- PMID: 23502512
- PMCID: PMC3652057
- DOI: 10.1152/ajplung.00370.2012
Combustion derived ultrafine particles induce cytochrome P-450 expression in specific lung compartments in the developing neonatal and adult rat
Abstract
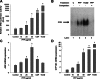
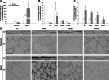
Vehicle exhaust is rich in polycyclic aromatic hydrocarbons (PAH) and can be a dominant contributor to ultrafine urban particulate matter (PM). Exposure to ultrafine PM is correlated with respiratory infections and asthmatic symptoms in young children. The lung undergoes substantial growth, alveolarization, and cellular maturation within the first years of life, which may be impacted by environmental pollutants such as PM. PAHs in PM can serve as ligands for the aryl hydrocarbon receptor (AhR) that induces expression of certain isozymes in the cytochrome P-450 superfamily, such as CYP1A1 and CYP1B1, localized in specific lung cell types. Although AhR activation and induction has been widely studied, its context within PM exposure and impact on the developing lung is poorly understood. In response, we have developed a replicable ultrafine premixed flame particle (PFP) generating system and used in vitro and in vivo models to define PM effects on AhR activation in the developing lung. We exposed 7-day neonatal and adult rats to a single 6-h PFP exposure and determined that PFPs cause significant parenchymal toxicity in neonates. PFPs contain weak AhR agonists that upregulate AhR-xenobiotic response element activity and expression and are capable inducers of CYP1A1 and CYP1B1 expression in both ages with different spatial and temporal patterns. Neonatal CYP1A1 expression was muted and delayed compared with adults, possibly because of differences in the enzyme maturation. We conclude that the inability of neonates to sufficiently adapt in response to PFP exposure may, in part, explain their susceptibility to PFP and urban ultrafine PM.
Keywords: aryl hydrocarbon receptor; lung development; particulate matter; polycyclic aromatic hydrocarbons.
Figures




Similar articles
-
Combustion-derived flame generated ultrafine soot generates reactive oxygen species and activates Nrf2 antioxidants differently in neonatal and adult rat lungs.Part Fibre Toxicol. 2013 Aug 1;10:34. doi: 10.1186/1743-8977-10-34. Part Fibre Toxicol. 2013. PMID: 23902943 Free PMC article.
-
Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice.Carcinogenesis. 2002 Jul;23(7):1199-207. doi: 10.1093/carcin/23.7.1199. Carcinogenesis. 2002. PMID: 12117779
-
Tissue-specific induction of cytochromes P450 1A1 and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in engineered C57BL/6J mice of arylhydrocarbon receptor gene.Toxicol Appl Pharmacol. 2003 Feb 15;187(1):1-10. doi: 10.1016/s0041-008x(02)00035-2. Toxicol Appl Pharmacol. 2003. PMID: 12628579
-
Lung cancer associated with combustion particles and fine particulate matter (PM2.5) - The roles of polycyclic aromatic hydrocarbons (PAHs) and the aryl hydrocarbon receptor (AhR).Biochem Pharmacol. 2023 Oct;216:115801. doi: 10.1016/j.bcp.2023.115801. Epub 2023 Sep 9. Biochem Pharmacol. 2023. PMID: 37696458 Free PMC article. Review.
-
Polycyclic aromatic hydrocarbon-aryl hydrocarbon receptor signaling regulates chronic inflammation in lung-gut axis.Toxicol Appl Pharmacol. 2025 Jul;500:117359. doi: 10.1016/j.taap.2025.117359. Epub 2025 May 2. Toxicol Appl Pharmacol. 2025. PMID: 40320014 Review.
Cited by
-
Transcriptomics profile of human bronchial epithelial cells exposed to ambient fine particles and influenza virus (H3N2).Sci Rep. 2023 Nov 7;13(1):19259. doi: 10.1038/s41598-023-46724-6. Sci Rep. 2023. PMID: 37935887 Free PMC article.
-
Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization.Toxicol Lett. 2018 Aug;292:85-96. doi: 10.1016/j.toxlet.2018.04.020. Epub 2018 Apr 22. Toxicol Lett. 2018. PMID: 29689377 Free PMC article.
-
Inhaled Pollutants: The Molecular Scene behind Respiratory and Systemic Diseases Associated with Ultrafine Particulate Matter.Int J Mol Sci. 2017 Jan 24;18(2):243. doi: 10.3390/ijms18020243. Int J Mol Sci. 2017. PMID: 28125025 Free PMC article. Review.
-
The Aryl Hydrocarbon Receptor (AHR): A Novel Therapeutic Target for Pulmonary Diseases?Int J Mol Sci. 2022 Jan 28;23(3):1516. doi: 10.3390/ijms23031516. Int J Mol Sci. 2022. PMID: 35163440 Free PMC article. Review.
-
Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats.Environ Sci Pollut Res Int. 2015 Dec;22(24):20167-76. doi: 10.1007/s11356-015-5222-z. Epub 2015 Aug 26. Environ Sci Pollut Res Int. 2015. PMID: 26304807
References
-
- Baker GL, Shultz MA, Fanucchi MV, Morin DM, Buckpitt AR, Plopper CG. Assessing gene expression in lung subcompartments utilizing in situ RNA preservation. Toxicol Sci 77: 135–141, 2004 - PubMed
-
- Branis M, Safranek J, Hytychova A. Exposure of children to airborne particulate matter of different size fractions during indoor physical education at school. Build Environ 44: 1246–1252, 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

