Copper metabolism of astrocytes
- PMID: 23503037
- PMCID: PMC3596760
- DOI: 10.3389/fnagi.2013.00009
Copper metabolism of astrocytes
Abstract
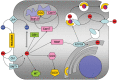
This short review will summarize the current knowledge on the uptake, storage, and export of copper ions by astrocytes and will address the potential roles of astrocytes in copper homeostasis in the normal and diseased brain. Astrocytes in culture efficiently accumulate copper by processes that include both the copper transporter Ctr1 and Ctr1-independent mechanisms. Exposure of astrocytes to copper induces an increase in cellular glutathione (GSH) content as well as synthesis of metallothioneins, suggesting that excess of copper is stored as complex with GSH and in metallothioneins. Furthermore, exposure of astrocytes to copper accelerates the release of GSH and glycolytically generated lactate. Astrocytes are able to export copper and express the Menkes protein ATP7A. This protein undergoes reversible, copper-dependent trafficking between the trans-Golgi network and vesicular structures. The ability of astrocytes to efficiently take up, store and export copper suggests that astrocytes play a key role in the supply of neurons with copper and that astrocytes should be considered as target for therapeutic interventions that aim to correct disturbances in brain copper homeostasis.
Keywords: ATP7A; Ctr1; astroglia; copper export; metallothioneins; oxidative stress; toxicity; transport.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

