Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation
- PMID: 23503106
- PMCID: PMC3596116
- DOI: 10.3762/bjoc.9.34
Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation
Abstract
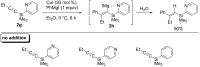
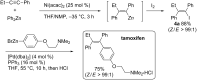
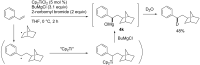
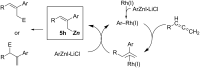
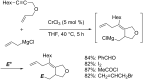
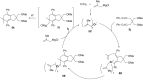
Carbomagnesiation and carbozincation reactions are efficient and direct routes to prepare complex and stereodefined organomagnesium and organozinc reagents. However, carbon-carbon unsaturated bonds are generally unreactive toward organomagnesium and organozinc reagents. Thus, transition metals were employed to accomplish the carbometalation involving wide varieties of substrates and reagents. Recent advances of transition-metal-catalyzed carbomagnesiation and carbozincation reactions are reviewed in this article. The contents are separated into five sections: carbomagnesiation and carbozincation of (1) alkynes bearing an electron-withdrawing group; (2) alkynes bearing a directing group; (3) strained cyclopropenes; (4) unactivated alkynes or alkenes; and (5) substrates that have two carbon-carbon unsaturated bonds (allenes, dienes, enynes, or diynes).
Keywords: alkene; alkyne; carbomagnesiation; carbometalation; carbozincation; transition metal.
Figures






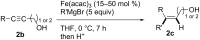













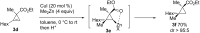





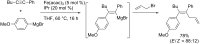








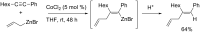


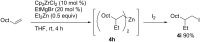












References
LinkOut - more resources
Full Text Sources
Other Literature Sources
