μ-opioid receptors in the stimulation of mesolimbic dopamine activity by ethanol and morphine in Long-Evans rats: a delayed effect of ethanol
- PMID: 23503684
- PMCID: PMC3707954
- DOI: 10.1007/s00213-013-3041-9
μ-opioid receptors in the stimulation of mesolimbic dopamine activity by ethanol and morphine in Long-Evans rats: a delayed effect of ethanol
Abstract
Rationale: Naltrexone, a non-selective opioid antagonist, decreases the euphoria and positive subjective responses to alcohol in heavy drinkers. It has been proposed that the μ-opioid receptor plays a role in ethanol reinforcement through modulation of ethanol-stimulated mesolimbic dopamine release.
Objectives: To investigate the ability of naltrexone and β-funaltrexamine, an irreversible μ-opioid specific antagonist, to inhibit ethanol-stimulated and morphine-stimulated mesolimbic dopamine release, and to determine whether opioid receptors on mesolimbic neurons contribute to these mechanisms.
Methods: Ethanol-naïve male Long Evans rats were given opioid receptor antagonists either intravenously, subcutaneously, or intracranially into the ventral tegmental area (VTA), followed by intravenous administration of ethanol or morphine. We measured extracellular dopamine in vivo using microdialysis probes inserted into the nucleus accumbens shell (n = 114).
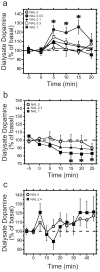
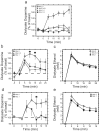
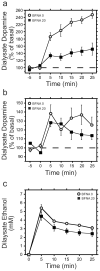
Results: Administration of naltrexone (intravenously) and β-funaltrexamine (subcutaneously), as well as intracranial injection of naltrexone into the VTA did not prevent the initiation of dopamine release by intravenous ethanol administration, but prevented it from being as prolonged. In contrast, morphine-stimulated mesolimbic dopamine release was effectively suppressed.
Conclusions: Our results provide novel evidence that there are two distinct mechanisms that mediate ethanol-stimulated mesolimbic dopamine release (an initial phase and a delayed phase), and that opioid receptor activation is required to maintain the delayed-phase dopamine release. Moreover, μ-opioid receptors account for this delayed-phase dopamine response, and the VTA is potentially the site of action of this mechanism. We conclude that μ-opioid receptors play different roles in the mechanisms of stimulation of mesolimbic dopamine activity by ethanol and morphine.
Figures


References
-
- Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Höllt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:296–302. - PubMed
-
- Blomqvist O, Engel JA, Nissbrandt H, Söderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249(2):207–13. - PubMed
-
- Borg PJ, Taylor DA. Involvement of mu- and delta-opioid receptors in the effects of systemic and locally perfused morphine on extracellular levels of dopamine, DOPAC and HVA in the nucleus accumbens of the halothane-anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:582–588. - PubMed
-
- Carboni E, Acquas E, Frau R, Di Chiara G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur J Pharmacol. 1989;164(3):515–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

