Primary aldosteronism and impaired natriuresis in mice underexpressing TGFβ1
- PMID: 23503843
- PMCID: PMC3619322
- DOI: 10.1073/pnas.1302641110
Primary aldosteronism and impaired natriuresis in mice underexpressing TGFβ1
Abstract
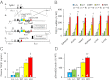
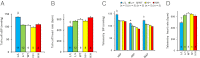
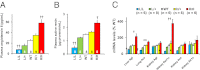
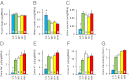
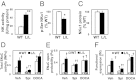
To uncover the potential cardiovascular effects of human polymorphisms influencing transforming growth factor β1 (TGFβ1) expression, we generated mice with Tgfb1 mRNA expression graded in five steps from 10% to 300% normal. Adrenal expression of the genes for mineralocorticoid-producing enzymes ranged from 50% normal in the hypermorphs at age 12 wk to 400% normal in the hypomorphs accompanied with proportionate changes in plasma aldosterone levels, whereas plasma volumes ranged from 50% to 150% normal accompanied by marked compensatory changes in plasma angiotensin II and renin levels. The aldosterone/renin ratio ranged from 0.3 times normal in the 300% hypermorphs to six times in the 10% hypomorphs, which have elevated blood pressure. Urinary output of water and electrolytes are markedly decreased in the 10% hypomorphs without significant change in the glomerular filtration rate. Renal activities for the Na(+), K(+)-ATPase, and epithelial sodium channel are markedly increased in the 10% hypomorphs. The hypertension in the 10% hypomorphs is corrected by spironolactone or amiloride at doses that do not change blood pressure in wild-type mice. Thus, changes in Tgfb1 expression cause marked progressive changes in multiple systems that regulate blood pressure and fluid homeostasis, with the major effects being mediated by changes in adrenocortical function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL071266/HL/NHLBI NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- HL71266/HL/NHLBI NIH HHS/United States
- U01 HL070523/HL/NHLBI NIH HHS/United States
- P30 DK056350/DK/NIDDK NIH HHS/United States
- DK056350/DK/NIDDK NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- DK34987/DK/NIDDK NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- R01 HL049277/HL/NHLBI NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- HL49277/HL/NHLBI NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- HL70523/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

