Regulation of iron metabolism by Pyrococcus furiosus
- PMID: 23504018
- PMCID: PMC3650548
- DOI: 10.1128/JB.02280-12
Regulation of iron metabolism by Pyrococcus furiosus
Abstract
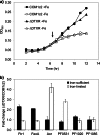
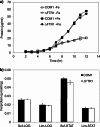
Iron is an essential element for the hyperthermophilic archaeon Pyrococcus furiosus, and many of its iron-containing enzymes have been characterized. How iron assimilation is regulated, however, is unknown. The genome sequence contains genes encoding two putative iron-responsive transcription factors, DtxR and Fur. Global transcriptional profiles of the dtxR deletion mutant (ΔDTXR) and the parent strain under iron-sufficient and iron-limited conditions indicated that DtxR represses the expression of the genes encoding two putative iron transporters, Ftr1 and FeoAB, under iron-sufficient conditions. Under iron limitation, DtxR represses expression of the gene encoding the iron-containing enzyme aldehyde ferredoxin oxidoreductase and a putative ABC-type transporter. Analysis of the dtxR gene sequence indicated an incorrectly predicted translation start site, and the corrected full-length DtxR protein, in contrast to the truncated version, specifically bound to the promoters of ftr1 and feoAB, confirming its role as a transcription regulator. Expression of the gene encoding Ftr1 was dramatically upregulated by iron limitation, but no phenotype was observed for the ΔFTR1 deletion mutant under iron-limited conditions. The intracellular iron concentrations of ΔFTR1 and the parent strain were similar, suggesting that under the conditions tested, Ftr1 is not an essential iron transporter despite its response to iron. In contrast to DtxR, the Fur protein appears not to be a functional regulator in P. furiosus, since it did not bind to the promoters of any of the iron-regulated genes and the deletion mutant (ΔFUR) revealed no transcriptional responses to iron availability. DtxR is therefore the key iron-responsive transcriptional regulator in P. furiosus.
Figures
Similar articles
-
The elemental sulfur-responsive protein (SipA) from the hyperthermophilic archaeon Pyrococcus furiosus is regulated by sulfide in an iron-dependent manner.J Bacteriol. 2010 Nov;192(21):5841-3. doi: 10.1128/JB.00660-10. Epub 2010 Aug 27. J Bacteriol. 2010. PMID: 20802041 Free PMC article.
-
The role of TrmB and TrmB-like transcriptional regulators for sugar transport and metabolism in the hyperthermophilic archaeon Pyrococcus furiosus.Arch Microbiol. 2008 Sep;190(3):247-56. doi: 10.1007/s00203-008-0378-2. Epub 2008 May 11. Arch Microbiol. 2008. PMID: 18470695 Review.
-
Purification and molecular characterization of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus: the third of a putative five-member tungstoenzyme family.J Bacteriol. 1999 Feb;181(4):1171-80. doi: 10.1128/JB.181.4.1171-1180.1999. J Bacteriol. 1999. PMID: 9973343 Free PMC article.
-
Characterization of the TrmB-like protein, PF0124, a TGM-recognizing global transcriptional regulator of the hyperthermophilic archaeon Pyrococcus furiosus.Mol Microbiol. 2007 Jul;65(2):305-18. doi: 10.1111/j.1365-2958.2007.05780.x. Epub 2007 Jun 21. Mol Microbiol. 2007. PMID: 17587231
-
The Development of Tungsten Biochemistry-A Personal Recollection.Molecules. 2023 May 11;28(10):4017. doi: 10.3390/molecules28104017. Molecules. 2023. PMID: 37241758 Free PMC article. Review.
Cited by
-
Comparative genomics of DtxR family regulons for metal homeostasis in Archaea.J Bacteriol. 2015 Feb;197(3):451-8. doi: 10.1128/JB.02386-14. Epub 2014 Nov 17. J Bacteriol. 2015. PMID: 25404694 Free PMC article.
-
Proteomic Analysis of Methanococcus voltae Grown in the Presence of Mineral and Nonmineral Sources of Iron and Sulfur.Microbiol Spectr. 2022 Aug 31;10(4):e0189322. doi: 10.1128/spectrum.01893-22. Epub 2022 Jul 25. Microbiol Spectr. 2022. PMID: 35876569 Free PMC article.
-
CopR, a Global Regulator of Transcription to Maintain Copper Homeostasis in Pyrococcus furiosus.Front Microbiol. 2021 Jan 11;11:613532. doi: 10.3389/fmicb.2020.613532. eCollection 2020. Front Microbiol. 2021. PMID: 33505379 Free PMC article.
-
A transcription network of interlocking positive feedback loops maintains intracellular iron balance in archaea.Nucleic Acids Res. 2017 Sep 29;45(17):9990-10001. doi: 10.1093/nar/gkx662. Nucleic Acids Res. 2017. PMID: 28973467 Free PMC article.
-
Global Transcriptional Programs in Archaea Share Features with the Eukaryotic Environmental Stress Response.J Mol Biol. 2019 Sep 20;431(20):4147-4166. doi: 10.1016/j.jmb.2019.07.029. Epub 2019 Aug 19. J Mol Biol. 2019. PMID: 31437442 Free PMC article. Review.
References
-
- Fiala G, Stetter KO. 1986. Pyrococcus furiosus sp. nov represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100-degrees C. Arch. Microbiol. 145:56–61
-
- Atomi H. 2005. Recent progress towards the application of hyperthermophiles and their enzymes. Curr. Opin. Chem. Biol. 9:166–173 - PubMed
-
- Verhaart MR, Bielen AA, van der Oost J, Stams AJ, Kengen SW. 2010. Hydrogen production by hyperthermophilic and extremely thermophilic bacteria and archaea: mechanisms for reductant disposal. Environ. Technol. 31:993–1003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

