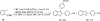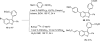Efficient Microwave-assisted One-pot Three-component Synthesis of 2,3-Disubstituted Benzofurans under Sonogashira Conditions
- PMID: 23504237
- PMCID: PMC3594699
- DOI: 10.1016/j.tet.2013.02.003
Efficient Microwave-assisted One-pot Three-component Synthesis of 2,3-Disubstituted Benzofurans under Sonogashira Conditions
Abstract
An efficient one-pot method for the synthesis of 2,3-disubstituted benzo[b]furans from commercially available 2-iodophenols, terminal acetylenes and aryl iodides has been developed utilizing Sonogashira reaction conditions. After an initial Sonogashira coupling of the 2-iodophenol with the terminal alkyne, cyclization involving the aryl iodide provides the 2,3-disubstituted benzo[b]furan in good to excellent yields. The use of microwave irradiation shortens the reaction times and minimizes the side products. This methodology is especially useful for the construction of libraries of highly substituted benzo[b]furans and their analogues.
Keywords: Sonogashira coupling; benzofuran; microwave; three-component reaction.
Figures
Similar articles
-
An Efficient, Microwave-Assisted, One-Pot Synthesis of Indoles Under Sonogashira Conditions.Tetrahedron. 2009 Oct 31;65(44):8908-8915. doi: 10.1016/j.tet.2009.07.075. Tetrahedron. 2009. PMID: 20160894 Free PMC article.
-
Facile one-pot synthesis of 4,5-disubstituted 1,2,3-(NH)-triazoles through Sonogashira coupling/1,3-dipolar cycloaddition of acid chlorides, terminal acetylenes, and sodium azide.Org Lett. 2009 Jul 16;11(14):3024-7. doi: 10.1021/ol901040d. Org Lett. 2009. PMID: 19537825
-
One-pot synthesis of substituted benzo[b]furans from mono- and dichlorophenols using palladium catalysts bearing dihydroxyterphenylphosphine.J Org Chem. 2013 Sep 20;78(18):9270-81. doi: 10.1021/jo401503t. Epub 2013 Aug 30. J Org Chem. 2013. PMID: 23952238
-
Ultrasound promoted copper-, ligand- and amine-free synthesis of benzo[b]furans/nitro benzo[b]furans via Sonogashira coupling-5-endo-dig-cyclization.Ultrason Sonochem. 2008 Jul;15(5):853-62. doi: 10.1016/j.ultsonch.2007.10.006. Epub 2007 Nov 4. Ultrason Sonochem. 2008. PMID: 18078777
-
Iodocyclization, followed by palladium-catalyzed coupling: a versatile strategy for heterocyclic library construction.Comb Chem High Throughput Screen. 2012 Jul;15(6):451-72. doi: 10.2174/138620712800563927. Comb Chem High Throughput Screen. 2012. PMID: 22272662 Review.
Cited by
-
Palladium-Catalyzed Synthesis of 2,3-Disubstituted Benzofurans: An Approach Towards the Synthesis of Deuterium Labeled Compounds.Adv Synth Catal. 2015 Jul 6;357(10):2331-2338. doi: 10.1002/adsc.201500308. Epub 2015 Jul 14. Adv Synth Catal. 2015. PMID: 26347405 Free PMC article.
-
Last Decade of Unconventional Methodologies for the Synthesis of Substituted Benzofurans.Molecules. 2020 May 16;25(10):2327. doi: 10.3390/molecules25102327. Molecules. 2020. PMID: 32429435 Free PMC article. Review.
-
Total Synthesis of Viniferifuran, Resveratrol-Piceatannol Hybrid, Anigopreissin A and Analogues - Investigation of Demethylation Strategies.Adv Synth Catal. 2016 Dec 22;358(24):4085-4092. doi: 10.1002/adsc.201601089. Epub 2016 Dec 8. Adv Synth Catal. 2016. PMID: 28701908 Free PMC article.
-
Nickel catalyzed intramolecular oxidative coupling: synthesis of 3-aryl benzofurans.RSC Adv. 2020 Jun 10;10(37):22264-22272. doi: 10.1039/d0ra03071f. eCollection 2020 Jun 8. RSC Adv. 2020. PMID: 35516592 Free PMC article.
-
Synthesis and Antimicrobial Activity of δ-Viniferin Analogues and Isosteres.Molecules. 2021 Dec 15;26(24):7594. doi: 10.3390/molecules26247594. Molecules. 2021. PMID: 34946674 Free PMC article.
References
-
-
For recent selected examples, see: Boto A, Alvarez L. Heterocycles in Nat. Prod. Synthesis. 2011:99–152.. Ashwood VA, Field MJ, Horwell DC, Julien-Larose C, Lewthwaite RA, McCleary S, Pritchard MC, Raphy J, Singh L. J. Med. Chem. 2001;44:2276–2285..
-
-
-
For some recent comprehensive reviews on the synthesis of benzofurans, see:Cacchi S, Fabrizi G, Goggiamani A. Org. Biomol. Chem. 2011;9:641–652.. Yeung K-S, Peng S-X, Wu J, Hou X-L. Prog. Heterocycl. Chem. 2011;23:195–229.. Wu J. Five-membered Heterocycles: Benzofuran and Related Systems. Modern Heterocyclic Chemistry; 2011. pp. 593–633.. Mentwally MA, Abdel-Wahab BF, Al-Hiti GA. Curr. Org. Chem. 2010;14:48–64.. Miyata O, Takeda N, Naito T. Heterocycles. 2009;78:843–871.. De Luca L, Nieddu G, Porcheddu A, Giacomelli G. Curr. Med. Chem. 2009;16:1–20. and references therein..
-
-
- Majumdar KC, Chattopadhyay B, Maji PK, Chattopadhyay SK, Samanta S. Heterocycles. 2010;81:517–584.
- Cacchi S, Fabrizi G, Goggiamani A. Curr. Org. Chem. 2006;10:1423–1455.
- Dudnik AS, Gevorgyan V. Catalyzed Carbon-Heteroatom Bond Formation. 2011:317–410.
-
d)For examples of Pd-catalyzed C-H activation leading to benzofuran formation, see:Ferreira EM, Zhang H, Stoltz BM. Tetrahedron. 2008;64:5987–6001..
-
- Arcadi A, Cacchi S, Del Rosario M, Fabrizi G, Marinelli F. J. Org. Chem. 1996;61:9280–9288.
-
- Chaplin JH, Flynn BL. Chem. Commun. 2001:1594–1595. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources