The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling
- PMID: 23504259
- PMCID: PMC3710311
- DOI: 10.1007/s10753-013-9621-3
The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling
Abstract
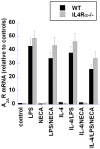
Murine macrophages are activated by interferon-γ (IFN-γ) and/or Toll-like receptor (TLR) agonists such as bacterial endotoxin (lipopolysaccharide [LPS]) to express an inflammatory (M1) phenotype characterized by the expression of nitric oxide synthase-2 (iNOS) and inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-12. In contrast, Th2 cytokines IL-4 and IL-13 activate macrophages by inducing the expression of arginase-1 and the anti-inflammatory cytokine IL-10 in an IL-4 receptor-α (IL-4Rα)-dependent manner. Macrophages activated in this way are designated as "alternatively activated" (M2a) macrophages. We have shown previously that adenosine A2A receptor (A(2A)R) agonists act synergistically with TLR2, TLR4, TLR7, and TLR9 agonists to switch macrophages into an "M2-like" phenotype that we have termed "M2d." Adenosine signaling suppresses the TLR-dependent expression of TNF-α, IL-12, IFN-γ, and several other inflammatory cytokines by macrophages and induces the expression of vascular endothelial growth factor (VEGF) and IL-10. We show here using mice lacking a functional IL-4Rα gene (IL-4Rα(-/-) mice) that this adenosine-mediated switch does not require IL-4Rα-dependent signaling. M2d macrophages express high levels of VEGF, IL-10, and iNOS, low levels of TNF-α and IL-12, and mildly elevated levels of arginase-1. In contrast, M2d macrophages do not express Ym1, Fizz1 (RELM-α), or CD206 at levels greater than those induced by LPS, and dectin-1 expression is suppressed. The use of these markers in vivo to identify "M2" macrophages thus provides an incomplete picture of macrophage functional status and should be viewed with caution.
Figures






Similar articles
-
IL-4Ralpha-independent expression of mannose receptor and Ym1 by macrophages depends on their IL-10 responsiveness.PLoS Negl Trop Dis. 2010 May 18;4(5):e689. doi: 10.1371/journal.pntd.0000689. PLoS Negl Trop Dis. 2010. PMID: 20502521 Free PMC article.
-
An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors.Am J Pathol. 2003 Aug;163(2):711-21. doi: 10.1016/S0002-9440(10)63698-X. Am J Pathol. 2003. PMID: 12875990 Free PMC article.
-
[Adipose-derived stem cells promote the polarization from M1 macrophages to M2 macrophages].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016 Mar;32(3):332-8. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016. PMID: 26927552 Chinese.
-
Shaping of monocyte and macrophage function by adenosine receptors.Pharmacol Ther. 2007 Feb;113(2):264-75. doi: 10.1016/j.pharmthera.2006.08.003. Epub 2006 Sep 14. Pharmacol Ther. 2007. PMID: 17056121 Free PMC article. Review.
-
Regulation of macrophage function by adenosine.Arterioscler Thromb Vasc Biol. 2012 Apr;32(4):865-9. doi: 10.1161/ATVBAHA.111.226852. Arterioscler Thromb Vasc Biol. 2012. PMID: 22423038 Free PMC article. Review.
Cited by
-
Type 1 interferon mediates chronic stress-induced neuroinflammation and behavioral deficits via complement component 3-dependent pathway.Mol Psychiatry. 2021 Jul;26(7):3043-3059. doi: 10.1038/s41380-021-01065-6. Epub 2021 Apr 8. Mol Psychiatry. 2021. PMID: 33833372 Free PMC article.
-
Acid external and internal environment exchange the Oreochromis niloticus tissue immune gene expression compared to the mouse macrophage polarization model.Front Immunol. 2022 Sep 26;13:1012078. doi: 10.3389/fimmu.2022.1012078. eCollection 2022. Front Immunol. 2022. PMID: 36225935 Free PMC article.
-
Landscape of adenosine pathway and immune checkpoint dual blockade in NSCLC: progress in basic research and clinical application.Front Immunol. 2024 Jan 29;15:1320244. doi: 10.3389/fimmu.2024.1320244. eCollection 2024. Front Immunol. 2024. PMID: 38348050 Free PMC article. Review.
-
Macrophage, a potential targeted therapeutic immune cell for cardiomyopathy.Front Cell Dev Biol. 2022 Sep 30;10:908790. doi: 10.3389/fcell.2022.908790. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36247005 Free PMC article. Review.
-
Pathophysiology of Atherosclerosis.Int J Mol Sci. 2022 Mar 20;23(6):3346. doi: 10.3390/ijms23063346. Int J Mol Sci. 2022. PMID: 35328769 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

