Technologies for investigating the physiological barriers to efficient lipid nanoparticle-siRNA delivery
- PMID: 23504369
- PMCID: PMC3715328
- DOI: 10.1369/0022155413484152
Technologies for investigating the physiological barriers to efficient lipid nanoparticle-siRNA delivery
Abstract
Small interfering RNA (siRNA) therapeutics have advanced from bench to clinical trials in recent years, along with new tools developed to enable detection of siRNA delivered at the organ, cell, and subcellular levels. Preclinical models of siRNA delivery have benefitted from methodologies such as stem-loop quantitative polymerase chain reaction, histological in situ immunofluorescent staining, endosomal escape assay, and RNA-induced silencing complex loading assay. These technologies have accelerated the detection and optimization of siRNA platforms to overcome the challenges associated with delivering therapeutic oligonucleotides to the cytosol of specific target cells. This review focuses on the methodologies and their application in the biodistribution of siRNA delivered by lipid nanoparticles.
Keywords: LNP; QWBA; RISC; biodistribution; delivery; endosomal escape; immunofluorescence staining; intravital imaging; lipid nanoparticle; siRNA; stem-loop PCR.
Conflict of interest statement
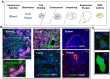
Figures

Similar articles
-
Quantitation of physiological and biochemical barriers to siRNA liver delivery via lipid nanoparticle platform.Mol Pharm. 2014 May 5;11(5):1424-34. doi: 10.1021/mp400584h. Epub 2014 Apr 1. Mol Pharm. 2014. PMID: 24588618
-
Understanding structure-activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo.J Control Release. 2019 Feb 10;295:140-152. doi: 10.1016/j.jconrel.2019.01.001. Epub 2019 Jan 2. J Control Release. 2019. PMID: 30610950
-
Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated delivery.J Histochem Cytochem. 2011 Aug;59(8):727-40. doi: 10.1369/0022155411410885. J Histochem Cytochem. 2011. PMID: 21804077 Free PMC article.
-
Lipid-based siRNA Delivery Systems: Challenges, Promises and Solutions Along the Long Journey.Curr Pharm Biotechnol. 2016;17(8):728-40. doi: 10.2174/1389201017666160401145319. Curr Pharm Biotechnol. 2016. PMID: 27033509 Review.
-
Multifunctional pH-Sensitive Amino Lipids for siRNA Delivery.Bioconjug Chem. 2016 Jan 20;27(1):19-35. doi: 10.1021/acs.bioconjchem.5b00538. Epub 2015 Dec 17. Bioconjug Chem. 2016. PMID: 26629982 Review.
Cited by
-
GL67 lipid-based liposomal formulation for efficient siRNA delivery into human lung cancer cells.Saudi Pharm J. 2023 Jul;31(7):1139-1148. doi: 10.1016/j.jsps.2023.05.017. Epub 2023 May 19. Saudi Pharm J. 2023. PMID: 37273265 Free PMC article.
-
Three-dimensional localization of polymer nanoparticles in cells using ToF-SIMS.Biointerphases. 2015 Jun 3;11(2):02A304. doi: 10.1116/1.4934795. Biointerphases. 2015. PMID: 26531772 Free PMC article.
-
Intratracheal Administration of siRNA Triggers mRNA Silencing in the Lung to Modulate T Cell Immune Response and Lung Inflammation.Mol Ther Nucleic Acids. 2019 Jun 7;16:194-205. doi: 10.1016/j.omtn.2019.02.013. Epub 2019 Feb 26. Mol Ther Nucleic Acids. 2019. PMID: 30901578 Free PMC article.
-
Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics.Adv Drug Deliv Rev. 2022 May;184:114236. doi: 10.1016/j.addr.2022.114236. Epub 2022 Mar 26. Adv Drug Deliv Rev. 2022. PMID: 35351470 Free PMC article. Review.
-
Current progress on aptamer-targeted oligonucleotide therapeutics.Ther Deliv. 2013 Dec;4(12):1527-46. doi: 10.4155/tde.13.118. Ther Deliv. 2013. PMID: 24304250 Free PMC article. Review.
References
-
- Akita H, Kogure K, Moriguchi R, Nakamura Y, Higashi T, Nakamura T, Serada S, Fujimoto M, Naka T, Futaki S, et al. 2010. Nanoparticles for ex vivo siRNA delivery to dendritic cells for cancer vaccines: programmed endosomal escape and dissociation. J Control Release. 143:311–317 - PubMed
-
- Aroeti B, Henis YI. 1991. Accumulation of Sendai virus glycoproteins in cell-cell contact regions and its role in cell fusion. J Biol Chem. 266:15845–15849 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

