Evaluation of serotonin 5-HT(1A) receptors in rodent models using [¹⁸F]mefway PET
- PMID: 23504990
- PMCID: PMC3744326
- DOI: 10.1002/syn.21665
Evaluation of serotonin 5-HT(1A) receptors in rodent models using [¹⁸F]mefway PET
Abstract
Introduction: Serotonin 5-HT(1A) receptors have been investigated in various CNS disorders, including epilepsy, mood disorders, and neurodegeneration. [¹⁸F]Mefway (N-{2-[4-(2'-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(cis/trans-4'-[¹⁸F]fluoromethylcyclohexane)-carboxamide) has been developed as a suitable positron emission tomography (PET) imaging agent for these receptors. We have now evaluated the suitability of [¹⁸F]trans-mefway in rat and mouse models using PET and computerized tomography (CT) imaging and corroborated with ex vivo and in vitro autoradiographic studies.
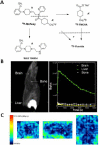
Methods: Normal Sprague-Dawley rats and Balb/C mice were used for PET/CT imaging using intravenously injected [¹⁸F]trans-mefway. Brain PET data were coregistered with rat and mouse magnetic resonance imaging template and regional distribution of radioactivity was quantitated. Selected animals were used for ex vivo autoradiographic studies to confirm regional brain distribution and quantitative measures of binding, using brain region to cerebellum ratios. Binding affinity of trans-mefway and WAY-100635 was measured in rat brain homogenates. Distribution of [¹⁸F]trans-4-fluoromethylcyclohexane carboxylate ([¹⁸F]FMCHA), a major metabolite of [¹⁸F] trans-mefway, was assessed in the rat by PET/CT.
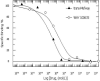
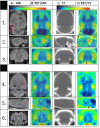
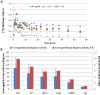
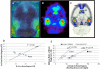
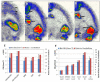
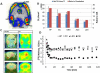
Results: The inhibition constant, K(i) for trans-mefway was 0.84 nM and that for WAY-100635 was 1.07 nM. Rapid brain uptake of [¹⁸F]trans-mefway was observed in all rat brain regions and clearance from cerebellum was fast and was used as a reference region in all studies. Distribution of [¹⁸F]trans-mefway in various brain regions was consistent in PET and in vitro studies. The dorsal raphe was visualized and quantified in the rat PET but identification in the mouse was difficult. The rank order of binding to the various brain regions was hippocampus > frontal cortex > anterior cingulate cortex > lateral septal nuclei > dorsal raphe nuclei.
Conclusion: [¹⁸F]trans-Mefway appears to be an effective 5-HT(1A) receptor imaging agent in rodents for studies of various disease models.
Keywords: 5-HT1A receptors; WAY-100635; mefway; microPET; serotonin.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
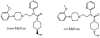
Similar articles
-
First-in-human evaluation of 18F-mefway, a PET radioligand specific to serotonin-1A receptors.J Nucl Med. 2014 Dec;55(12):1973-9. doi: 10.2967/jnumed.114.145151. Epub 2014 Nov 13. J Nucl Med. 2014. PMID: 25453045 Free PMC article.
-
Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, 18F-labeled mefway, in rodents and imaging by PET in a nonhuman primate.J Nucl Med. 2006 Oct;47(10):1697-706. J Nucl Med. 2006. PMID: 17015907
-
Comparative assessment of (18) F-Mefway as a serotonin 5-HT1A receptor PET imaging agent across species: Rodents, nonhuman primates, and humans.J Comp Neurol. 2016 May 1;524(7):1457-71. doi: 10.1002/cne.23919. Epub 2015 Nov 18. J Comp Neurol. 2016. PMID: 26509362 Free PMC article.
-
N-{2-[4-(2-Methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-[18F]-fluoromethylcyclohexane)carboxamide.2006 Nov 9 [updated 2012 Apr 11]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2006 Nov 9 [updated 2012 Apr 11]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641628 Free Books & Documents. Review.
-
PET tracers for 5-HT(1A) receptors and uses thereof.Drug Discov Today. 2007 Sep;12(17-18):748-56. doi: 10.1016/j.drudis.2007.07.008. Epub 2007 Aug 27. Drug Discov Today. 2007. PMID: 17826688 Review.
Cited by
-
First-in-human evaluation of 18F-mefway, a PET radioligand specific to serotonin-1A receptors.J Nucl Med. 2014 Dec;55(12):1973-9. doi: 10.2967/jnumed.114.145151. Epub 2014 Nov 13. J Nucl Med. 2014. PMID: 25453045 Free PMC article.
-
Evidence For Cannabidiol Modulation of Serotonergic Transmission in a Model of Osteoarthritis via in vivo PET Imaging and Behavioral Assessment.Int J Innov Res Med Sci. 2022 Jun;7(6):254-271. doi: 10.23958/ijirms/vol07-i06/1418. Epub 2022 Jun 3. Int J Innov Res Med Sci. 2022. PMID: 37841504 Free PMC article.
-
Synthesis and Evaluation of Mefway Analogs as Ligands for Serotonin 5HT1A Receptors.Med Chem Res. 2015 Apr;24(4):1480-1486. doi: 10.1007/s00044-014-1238-z. Med Chem Res. 2015. PMID: 25750500 Free PMC article.
-
18F-Mefway PET imaging of serotonin 1A receptors in humans: a comparison with 18F-FCWAY.PLoS One. 2015 Apr 1;10(4):e0121342. doi: 10.1371/journal.pone.0121342. eCollection 2015. PLoS One. 2015. PMID: 25830772 Free PMC article.
-
Synthesis and evaluation of (S)-[(18)F]fesetron in the rat brain as a potential PET imaging agent for serotonin 5-HT3 receptors.Bioorg Med Chem Lett. 2016 Apr 15;26(8):1919-24. doi: 10.1016/j.bmcl.2016.03.018. Epub 2016 Mar 8. Bioorg Med Chem Lett. 2016. PMID: 26979158 Free PMC article.
References
-
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol. Psychiatry. 2009;66:627–635. - PubMed
-
- Assem-Hilger E, Lanzenberger R, Savli M, Wadsak W, Mitterhauser M, Mien LK, Stogmann E, Baumgartner C, Kletter K, Asenbaum S. Central serotonin 1A receptor binding in temporal lobe epilepsy: A carbonyl-11C-WAY-100635 PET study. Epilepsy Behav. 2010;19:467–473. - PubMed
-
- Bellido I, Diaz-Cabiale Z, Jimenez-Vasquez PA, Andbjer B, Mathe AA, Fuxe K. Increased density of galanin binding sites in the dorsal raphe in a genetic rat modelo f depression. Neurosci. Lett. 2002;11:101–105. - PubMed
-
- Bert B, Fink H, Rothe J, Walstab J, Bonisch H. Learning and memory in 5-HT1A receptor mutant mice. Behav Brain Res. 2008;195:78–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
