Gene regulatory networks mediating canonical Wnt signal-directed control of pluripotency and differentiation in embryo stem cells
- PMID: 23505158
- PMCID: PMC3830733
- DOI: 10.1002/stem.1371
Gene regulatory networks mediating canonical Wnt signal-directed control of pluripotency and differentiation in embryo stem cells
Abstract
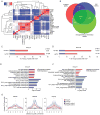
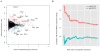
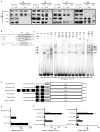
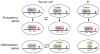
Canonical Wnt signaling supports the pluripotency of embryonic stem cells (ESCs) but also promotes differentiation of early mammalian cell lineages. To explain these paradoxical observations, we explored the gene regulatory networks at play. Canonical Wnt signaling is intertwined with the pluripotency network comprising Nanog, Oct4, and Sox2 in mouse ESCs. In defined media supporting the derivation and propagation of ESCs, Tcf3 and β-catenin interact with Oct4; Tcf3 binds to Sox motif within Oct-Sox composite motifs that are also bound by Oct4-Sox2 complexes. Furthermore, canonical Wnt signaling upregulates the activity of the Pou5f1 distal enhancer via the Sox motif in ESCs. When viewed in the context of published studies on Tcf3 and β-catenin mutants, our findings suggest Tcf3 counters pluripotency by competition with Sox2 at these sites, and Tcf3 inhibition is blocked by β-catenin entry into this complex. Wnt pathway stimulation also triggers β-catenin association at regulatory elements with classic Lef/Tcf motifs associated with differentiation programs. The failure to activate these targets in the presence of a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) inhibitor essential for ESC culture suggests MEK/ERK signaling and canonical Wnt signaling combine to promote ESC differentiation.
Keywords: Differentiation; Mouse embryonic stem cells; Pluripotency; Wnt; β-catenin.
© AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures



References
-
- Loh Y-H, Wu Q, Chew J-L, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. - PubMed
-
- Jiang J, Chan Y-S, Loh Y-H, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. - PubMed
-
- Gonzalez F, Boue S, Belmonte JCI. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nature Reviews Genetics. 2011;12:231–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

