Acyl-lipid metabolism
- PMID: 23505340
- PMCID: PMC3563272
- DOI: 10.1199/tab.0161
Acyl-lipid metabolism
Abstract
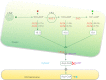
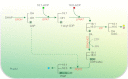
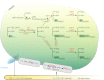
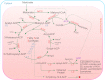
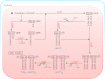
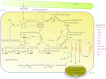
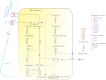
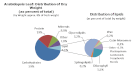
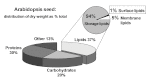
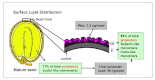
Acyl lipids in Arabidopsis and all other plants have a myriad of diverse functions. These include providing the core diffusion barrier of the membranes that separates cells and subcellular organelles. This function alone involves more than 10 membrane lipid classes, including the phospholipids, galactolipids, and sphingolipids, and within each class the variations in acyl chain composition expand the number of structures to several hundred possible molecular species. Acyl lipids in the form of triacylglycerol account for 35% of the weight of Arabidopsis seeds and represent their major form of carbon and energy storage. A layer of cutin and cuticular waxes that restricts the loss of water and provides protection from invasions by pathogens and other stresses covers the entire aerial surface of Arabidopsis. Similar functions are provided by suberin and its associated waxes that are localized in roots, seed coats, and abscission zones and are produced in response to wounding. This chapter focuses on the metabolic pathways that are associated with the biosynthesis and degradation of the acyl lipids mentioned above. These pathways, enzymes, and genes are also presented in detail in an associated website (ARALIP: http://aralip.plantbiology.msu.edu/). Protocols and methods used for analysis of Arabidopsis lipids are provided. Finally, a detailed summary of the composition of Arabidopsis lipids is provided in three figures and 15 tables.
Figures
















References
-
- Adham A.R., Zolman B.K., Millius A., Bartel B. Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in beta-oxidation. Plant J. 2005;41:859–874. - PubMed
-
- Agrawal V.P., Kolattukudy P.E. Purification and characterization of a wound-induced omega-hydroxyfatty acid:NADP oxidoreductase from potato tuber disks (Solanum tuberosum L.). Arch. Biochem. Biophys. 1978;191:452–465. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
