Analysis of the membrane proteome of ciprofloxacin-resistant macrophages by stable isotope labeling with amino acids in cell culture (SILAC)
- PMID: 23505477
- PMCID: PMC3591400
- DOI: 10.1371/journal.pone.0058285
Analysis of the membrane proteome of ciprofloxacin-resistant macrophages by stable isotope labeling with amino acids in cell culture (SILAC)
Abstract
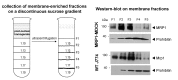
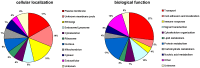
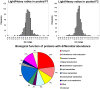
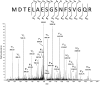
Overexpression of multidrug transporters is a well-established mechanism of resistance to chemotherapy, but other changes may be co-selected upon exposure to drugs that contribute to resistance. Using a model of J774 macrophages made resistant to the fluoroquinolone antibiotic ciprofloxacin and comparing it with the wild-type parent cell line, we performed a quantitative proteomic analysis using the stable isotope labeling with amino acids in cell culture technology coupled with liquid chromatography electrospray ionization Fourier transform tandem mass spectrometry (LC-ESI-FT-MS/MS) on 2 samples enriched in membrane proteins (fractions F1 and F2 collected from discontinuous sucrose gradient). Nine hundred proteins were identified with at least 3 unique peptides in these 2 pooled fractions among which 61 (F1) and 69 (F2) showed a significantly modified abundance among the 2 cell lines. The multidrug resistance associated protein Abcc4, known as the ciprofloxacin efflux transporter in these cells, was the most upregulated, together with Dnajc3, a protein encoded by a gene located downstream of Abcc4. The other modulated proteins are involved in transport functions, cell adhesion and cytoskeleton organization, immune response, signal transduction, and metabolism. This indicates that the antibiotic ciprofloxacin is able to trigger a pleiotropic adaptative response in macrophages that includes the overexpression of its efflux transporter.
Conflict of interest statement
Figures


References
-
- Dean M, Rzhetsky A, Allikmets R (2001) The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 11: 1156–1166. - PubMed
-
- Deeley RG, Westlake C, Cole SP (2006) Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86: 849–899. - PubMed
-
- Borst P, Jonkers J, Rottenberg S (2007) What makes tumors multidrug resistant? Cell Cycle 6: 2782–2787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

