High efficiency diffusion molecular retention tumor targeting
- PMID: 23505478
- PMCID: PMC3594319
- DOI: 10.1371/journal.pone.0058290
High efficiency diffusion molecular retention tumor targeting
Abstract
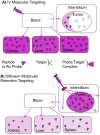
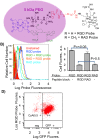
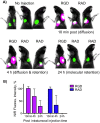
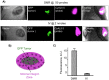
Here we introduce diffusion molecular retention (DMR) tumor targeting, a technique that employs PEG-fluorochrome shielded probes that, after a peritumoral (PT) injection, undergo slow vascular uptake and extensive interstitial diffusion, with tumor retention only through integrin molecular recognition. To demonstrate DMR, RGD (integrin binding) and RAD (control) probes were synthesized bearing DOTA (for (111) In(3+)), a NIR fluorochrome, and 5 kDa PEG that endows probes with a protein-like volume of 25 kDa and decreases non-specific interactions. With a GFP-BT-20 breast carcinoma model, tumor targeting by the DMR or i.v. methods was assessed by surface fluorescence, biodistribution of [(111)In] RGD and [(111)In] RAD probes, and whole animal SPECT. After a PT injection, both probes rapidly diffused through the normal and tumor interstitium, with retention of the RGD probe due to integrin interactions. With PT injection and the [(111)In] RGD probe, SPECT indicated a highly tumor specific uptake at 24 h post injection, with 352%ID/g tumor obtained by DMR (vs 4.14%ID/g by i.v.). The high efficiency molecular targeting of DMR employed low probe doses (e.g. 25 ng as RGD peptide), which minimizes toxicity risks and facilitates clinical translation. DMR applications include the delivery of fluorochromes for intraoperative tumor margin delineation, the delivery of radioisotopes (e.g. toxic, short range alpha emitters) for radiotherapy, or the delivery of photosensitizers to tumors accessible to light.
Conflict of interest statement
Figures


Similar articles
-
Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor alphavbeta3-integrin expression.J Nucl Med. 2004 Oct;45(10):1776-83. J Nucl Med. 2004. PMID: 15471848
-
The PEG-fluorochrome shielding approach for targeted probe design.J Am Chem Soc. 2012 Nov 28;134(47):19338-41. doi: 10.1021/ja309085b. Epub 2012 Nov 14. J Am Chem Soc. 2012. PMID: 23137147 Free PMC article.
-
Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature.J Nucl Med. 2007 Nov;48(11):1862-70. doi: 10.2967/jnumed.107.043216. Epub 2007 Oct 17. J Nucl Med. 2007. PMID: 17942800
-
Fast clearing RGD-based near-infrared fluorescent probes for in vivo tumor diagnosis.Contrast Media Mol Imaging. 2012 Jul-Aug;7(4):390-402. doi: 10.1002/cmmi.1464. Contrast Media Mol Imaging. 2012. PMID: 22649045
-
Integrin (αvβ3) Targeted RGD Peptide Based Probe for Cancer Optical Imaging.Curr Protein Pept Sci. 2016;17(6):570-81. doi: 10.2174/1389203717666160101124015. Curr Protein Pept Sci. 2016. PMID: 26721402 Review.
Cited by
-
Heat-induced-radiolabeling and click chemistry: A powerful combination for generating multifunctional nanomaterials.PLoS One. 2017 Feb 22;12(2):e0172722. doi: 10.1371/journal.pone.0172722. eCollection 2017. PLoS One. 2017. PMID: 28225818 Free PMC article.
-
Near-infrared fluorescent probes in cancer imaging and therapy: an emerging field.Int J Nanomedicine. 2014 Mar 5;9:1347-65. doi: 10.2147/IJN.S60206. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24648733 Free PMC article. Review.
-
Peptides in receptor-mediated radiotherapy: from design to the clinical application in cancers.Front Oncol. 2013 Sep 25;3:247. doi: 10.3389/fonc.2013.00247. Front Oncol. 2013. PMID: 24093086 Free PMC article. Review.
-
PEG-like nanoprobes: multimodal, pharmacokinetically and optically tunable nanomaterials.PLoS One. 2014 Apr 29;9(4):e95406. doi: 10.1371/journal.pone.0095406. eCollection 2014. PLoS One. 2014. PMID: 24781778 Free PMC article.
-
Recent developments in multimodality fluorescence imaging probes.Acta Pharm Sin B. 2018 May;8(3):320-338. doi: 10.1016/j.apsb.2018.03.010. Epub 2018 Mar 30. Acta Pharm Sin B. 2018. PMID: 29881672 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

