Differential activation of killer cells in the circulation and the lung: a study of current smoking status and chronic obstructive pulmonary disease (COPD)
- PMID: 23505535
- PMCID: PMC3594304
- DOI: 10.1371/journal.pone.0058556
Differential activation of killer cells in the circulation and the lung: a study of current smoking status and chronic obstructive pulmonary disease (COPD)
Abstract
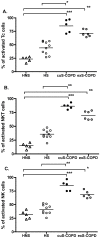
Background: CD8(+) T-lymphocytes, natural killer T-like cells (NKT-like cells, CD56(+)CD3(+)) and natural killer cells (NK cells, CD56(+)CD3(-)) are the three main classes of human killer cells and they are implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD). Activation of these cells can initiate immune responses by virtue of their production of inflammatory cytokines and chemokines that cause lung tissue damage, mucus hypersecretion and emphysema. The objective of the current study was to investigate the activation levels of human killer cells in healthy non-smokers, healthy smokers, ex-smokers with COPD and current smokers with COPD, in both peripheral blood and induced sputum.
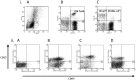
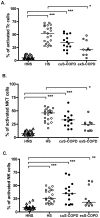
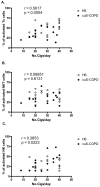
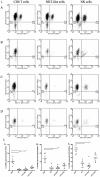
Methods/principal findings: After informed consent, 124 participants were recruited into the study and peripheral blood or induced sputum was taken. The activation states and receptor expression of killer cells were measured by flow cytometry. In peripheral blood, current smokers, regardless of disease state, have the highest proportion of activated CD8(+) T-lymphocytes, NKT-like cells and NK cells compared with ex-smokers with COPD and healthy non-smokers. Furthermore, CD8(+) T-lymphocyte and NK cell activation is positively correlated with the number of cigarettes currently smoked. Conversely, in induced sputum, the proportion of activated killer cells was related to disease state rather than current smoking status, with current and ex-smokers with COPD having significantly higher rates of activation than healthy smokers and healthy non-smokers.
Conclusions: A differential effect in systemic and lung activation of killer cells in COPD is evident. Systemic activation appears to be related to current smoking whereas lung activation is related to the presence or absence of COPD, irrespective of current smoking status. These findings suggest that modulating killer cell activation may be a new target for the treatment of COPD.
Conflict of interest statement
Figures
Similar articles
-
Enhanced effector function of cytotoxic cells in the induced sputum of COPD patients.Respir Res. 2010 Jun 11;11(1):76. doi: 10.1186/1465-9921-11-76. Respir Res. 2010. PMID: 20540777 Free PMC article.
-
Enhanced cytotoxic function of natural killer and natural killer T-like cells associated with decreased CD94 (Kp43) in the chronic obstructive pulmonary disease airway.Respirology. 2013 Feb;18(2):369-76. doi: 10.1111/j.1440-1843.2012.02287.x. Respirology. 2013. PMID: 23062183
-
Sustained CTL activation by murine pulmonary epithelial cells promotes the development of COPD-like disease.J Clin Invest. 2009 Mar;119(3):636-49. doi: 10.1172/JCI34462. Epub 2009 Feb 9. J Clin Invest. 2009. PMID: 19197141 Free PMC article.
-
Killer cells in chronic obstructive pulmonary disease.Clin Sci (Lond). 2008 Apr;114(8):533-41. doi: 10.1042/CS20070356. Clin Sci (Lond). 2008. PMID: 18336369 Review.
-
NK Cells in the Pathogenesis of Chronic Obstructive Pulmonary Disease.Front Immunol. 2021 May 4;12:666045. doi: 10.3389/fimmu.2021.666045. eCollection 2021. Front Immunol. 2021. PMID: 34017339 Free PMC article. Review.
Cited by
-
Lipid-Reactive T Cells in Immunological Disorders of the Lung.Front Immunol. 2018 Sep 26;9:2205. doi: 10.3389/fimmu.2018.02205. eCollection 2018. Front Immunol. 2018. PMID: 30319649 Free PMC article. Review.
-
Roles of Myeloid and Lymphoid Cells in the Pathogenesis of Chronic Obstructive Pulmonary Disease.Front Immunol. 2018 Jun 21;9:1431. doi: 10.3389/fimmu.2018.01431. eCollection 2018. Front Immunol. 2018. PMID: 29977245 Free PMC article. Review.
-
Evidence construction of Jinshuibao capsules against stable chronic obstructive pulmonary disease: A systematic review and network pharmacology.Heliyon. 2024 Jul 14;10(14):e34572. doi: 10.1016/j.heliyon.2024.e34572. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39082031 Free PMC article.
-
The Influence of Innate Lymphoid Cells and Unconventional T Cells in Chronic Inflammatory Lung Disease.Front Immunol. 2019 Jul 11;10:1597. doi: 10.3389/fimmu.2019.01597. eCollection 2019. Front Immunol. 2019. PMID: 31354734 Free PMC article. Review.
-
A Meta-Analysis of the Performance of a Blood-Based Exposure Response Gene Signature Across Clinical Studies on the Tobacco Heating System 2.2 (THS 2.2).Front Pharmacol. 2019 Mar 26;10:198. doi: 10.3389/fphar.2019.00198. eCollection 2019. Front Pharmacol. 2019. PMID: 30971916 Free PMC article.
References
-
- Celli BR, MacNee W (2004) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23: 932–946. - PubMed
-
- Agusti A, Barnes PJ (2012) Update in chronic obstructive pulmonary disease 2011. American journal of respiratory and critical care medicine 185: 1171–1176. - PubMed
-
- Knight DA, Yang IA, Ko FW, Lim TK (2012) Year in review 2011: asthma, chronic obstructive pulmonary disease and airway biology. Respirology 17: 563–572. - PubMed
-
- Pauwels RA, Rabe KF (2004) Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 364: 613–620. - PubMed
-
- Mannino DM, Buist AS (2007) Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370: 765–773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

