Combined delivery of paclitaxel and tanespimycin via micellar nanocarriers: pharmacokinetics, efficacy and metabolomic analysis
- PMID: 23505544
- PMCID: PMC3591361
- DOI: 10.1371/journal.pone.0058619
Combined delivery of paclitaxel and tanespimycin via micellar nanocarriers: pharmacokinetics, efficacy and metabolomic analysis
Abstract
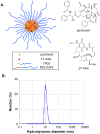
Background: Despite the promising anticancer efficacy observed in preclinical studies, paclitaxel and tanespimycin (17-AAG) combination therapy has yielded meager responses in a phase I clinical trial. One serious problem associated with paclitaxel/17-AAG combination therapy is the employment of large quantities of toxic organic surfactants and solvents for drug solubilization. The goal of this study was to evaluate a micellar formulation for the concurrent delivery of paclitaxel and 17-AAG in vivo.
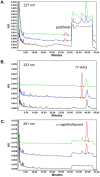
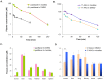
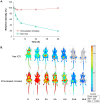
Methodology/principal findings: Paclitaxel/17-AAG-loaded micelles were assessed in mice bearing human ovarian tumor xenografts. Compared with the free drugs at equivalent doses, intravenous administration of paclitaxel/17-AAG-loaded micelles led to 3.5- and 1.7-fold increase in the tumor concentrations of paclitaxel and 17-AAG, respectively, without significant altering drug levels in normal organs. The enhanced tumor accumulation of the micellar drugs was further confirmed by the whole-body near infrared imaging using indocyanine green-labeled micelles. Subsequently, the anticancer efficacy of paclitaxel/17-AAG-loaded micelles was examined in comparison with the free drugs (weekly 20 mg/kg paclitaxel, twice-weekly 37.5 mg/kg 17-AAG). We found that paclitaxel/17-AAG-loaded micelles caused near-complete arrest of tumor growth, whereas the free drug-treated tumors experienced rapid growth shortly after the 3-week treatment period ended. Furthermore, comparative metabolomic profiling by proton nuclear magnetic resonance revealed significant decrease in glucose, lactate and alanine with simultaneous increase in glutamine, glutamate, aspartate, choline, creatine and acetate levels in the tumors of mice treated with paclitaxel/17-AAG-loaded micelles.
Conclusions/significance: We have demonstrated in the current wok a safe and efficacious nano-sized formulation for the combined delivery of paclitaxel and 17-AAG, and uncovered unique metabolomic signatures in the tumor that correlate with the favorable therapeutic response to paclitaxel/17-AAG combination therapy.
Conflict of interest statement
Figures

Similar articles
-
Multi-drug delivery to tumor cells via micellar nanocarriers.Int J Pharm. 2011 Oct 31;419(1-2):281-6. doi: 10.1016/j.ijpharm.2011.07.033. Epub 2011 Jul 27. Int J Pharm. 2011. PMID: 21820041 Free PMC article.
-
Pharmacokinetic study of 3-in-1 poly(ethylene glycol)-block-poly(D, L-lactic acid) micelles carrying paclitaxel, 17-allylamino-17-demethoxygeldanamycin, and rapamycin.J Control Release. 2012 Oct 10;163(1):93-9. doi: 10.1016/j.jconrel.2012.04.024. Epub 2012 Apr 23. J Control Release. 2012. PMID: 22549011 Free PMC article.
-
A cremophor-free formulation for tanespimycin (17-AAG) using PEO-b-PDLLA micelles: characterization and pharmacokinetics in rats.J Pharm Sci. 2009 Apr;98(4):1577-86. doi: 10.1002/jps.21509. J Pharm Sci. 2009. PMID: 18752263 Free PMC article.
-
Synthesis and evaluation of poly(styrene-co-maleic acid) micellar nanocarriers for the delivery of tanespimycin.Int J Pharm. 2011 Nov 25;420(1):111-7. doi: 10.1016/j.ijpharm.2011.08.011. Epub 2011 Aug 12. Int J Pharm. 2011. PMID: 21856392 Free PMC article.
-
Spotlight on 17-AAG as an Hsp90 inhibitor for molecular targeted cancer treatment.Chem Biol Drug Des. 2019 May;93(5):760-786. doi: 10.1111/cbdd.13486. Epub 2019 Feb 14. Chem Biol Drug Des. 2019. PMID: 30697932 Review.
Cited by
-
Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer.Nanomaterials (Basel). 2021 Apr 20;11(4):1048. doi: 10.3390/nano11041048. Nanomaterials (Basel). 2021. PMID: 33923947 Free PMC article. Review.
-
Reduction-responsive crosslinked micellar nanoassemblies for tumor-targeted drug delivery.Pharm Res. 2015 Apr;32(4):1325-40. doi: 10.1007/s11095-014-1537-6. Epub 2014 Oct 16. Pharm Res. 2015. PMID: 25319102
-
Exploring polymeric micelles for improved delivery of anticancer agents: recent developments in preclinical studies.Pharmaceutics. 2013 Mar 22;5(1):201-19. doi: 10.3390/pharmaceutics5010201. Pharmaceutics. 2013. PMID: 24300405 Free PMC article.
-
Triblock-Diblock Composite Nanoassemblies with Sequentially Addressable Host-Guest Properties for Hydrophobics and Hydrophilics.ACS Macro Lett. 2020 Jun 23;9(7):1019-1023. doi: 10.1021/acsmacrolett.0c00290. ACS Macro Lett. 2020. PMID: 34268003 Free PMC article.
-
Cancer nanomedicine: from targeted delivery to combination therapy.Trends Mol Med. 2015 Apr;21(4):223-32. doi: 10.1016/j.molmed.2015.01.001. Epub 2015 Feb 2. Trends Mol Med. 2015. PMID: 25656384 Free PMC article. Review.
References
-
- Davis ME, Chen Z, Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7: 771–782. - PubMed
-
- Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res 46: 6387–6392. - PubMed
-
- Aryal S, Hu C-MJ, Zhang L (2010) Combinatorial drug conjugation enables nanoparticle dual drug delivery. Small 6: 1442–1448. - PubMed
-
- Dicko A, Mayer LD, Tardi PG (2010) Use of nanoscale delivery systems to maintain synergistic drug ratios in vivo . Expert Opin Drug Deliv 7: 1329–1341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

