Alterations in peripheral blood B cell subsets and dynamics of B cell responses during human schistosomiasis
- PMID: 23505586
- PMCID: PMC3591311
- DOI: 10.1371/journal.pntd.0002094
Alterations in peripheral blood B cell subsets and dynamics of B cell responses during human schistosomiasis
Abstract
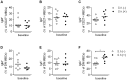
Antibody responses are thought to play an important role in control of Schistosoma infections, yet little is known about the phenotype and function of B cells in human schistosomiasis. We set out to characterize B cell subsets and B cell responses to B cell receptor and Toll-like receptor 9 stimulation in Gabonese schoolchildren with Schistosoma haematobium infection. Frequencies of memory B cell (MBC) subsets were increased, whereas naive B cell frequencies were reduced in the schistosome-infected group. At the functional level, isolated B cells from schistosome-infected children showed higher expression of the activation marker CD23 upon stimulation, but lower proliferation and TNF-α production. Importantly, 6-months after 3 rounds of praziquantel treatment, frequencies of naive B cells were increased, MBC frequencies were decreased and with the exception of TNF-α production, B cell responsiveness was restored to what was seen in uninfected children. These data show that S. haematobium infection leads to significant changes in the B cell compartment, both at the phenotypic and functional level.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
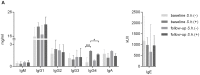




Similar articles
-
Population level changes in schistosome-specific antibody levels following chemotherapy.Parasite Immunol. 2019 Jan;41(1):e12604. doi: 10.1111/pim.12604. Parasite Immunol. 2019. PMID: 30467873 Free PMC article.
-
A Praziquantel Treatment Study of Immune and Transcriptome Profiles in Schistosoma haematobium-Infected Gabonese Schoolchildren.J Infect Dis. 2020 Nov 13;222(12):2103-2113. doi: 10.1093/infdis/jiz641. J Infect Dis. 2020. PMID: 31844885 Free PMC article.
-
Benefits of annual chemotherapeutic control of schistosomiasis on the development of protective immunity.BMC Infect Dis. 2019 Mar 4;19(1):219. doi: 10.1186/s12879-019-3811-z. BMC Infect Dis. 2019. PMID: 30832614 Free PMC article.
-
Integrated analysis of innate, Th1, Th2, Th17, and regulatory cytokines identifies changes in immune polarisation following treatment of human schistosomiasis.J Infect Dis. 2013 Jul;208(1):159-69. doi: 10.1093/infdis/jis524. Epub 2012 Oct 8. J Infect Dis. 2013. PMID: 23045617 Free PMC article.
-
Helminth parasite proteomics: from experimental models to human infections.Parasitology. 2012 Aug;139(9):1195-204. doi: 10.1017/S0031182011002423. Epub 2012 Mar 28. Parasitology. 2012. PMID: 22455721 Free PMC article. Review.
Cited by
-
Schistosome egg antigens, including the glycoprotein IPSE/alpha-1, trigger the development of regulatory B cells.PLoS Pathog. 2017 Jul 28;13(7):e1006539. doi: 10.1371/journal.ppat.1006539. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28753651 Free PMC article.
-
CD23 can negatively regulate B-cell receptor signaling.Sci Rep. 2016 May 16;6:25629. doi: 10.1038/srep25629. Sci Rep. 2016. PMID: 27181049 Free PMC article.
-
Subversion of the B-cell compartment during parasitic, bacterial, and viral infections.BMC Immunol. 2015 Mar 26;16:15. doi: 10.1186/s12865-015-0079-y. BMC Immunol. 2015. PMID: 25884828 Free PMC article. Review.
-
Worms: Pernicious parasites or allies against allergies?Parasite Immunol. 2019 Jun;41(6):e12574. doi: 10.1111/pim.12574. Epub 2018 Aug 29. Parasite Immunol. 2019. PMID: 30043455 Free PMC article. Review.
-
Multi-dimensional analysis of B cells reveals the expansion of memory and regulatory B-cell clusters in humans living in rural tropical areas.Clin Exp Immunol. 2025 Jan 21;219(1):uxae074. doi: 10.1093/cei/uxae074. Clin Exp Immunol. 2025. PMID: 39129562 Free PMC article.
References
-
- World Health Organization (2012) Schistosomiasis. Available: http://www.who.int/mediacentre/factsheets/fs115/en/index.html. Accessed 26 June 2012.
-
- Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, et al. (2009) Immunopathogenesis of human schistosomiasis. Parasite Immunol 31: 163–176. - PubMed
-
- van Riet E, Hartgers FC, Yazdanbakhsh M (2007) Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212: 475–490. - PubMed
-
- Allen JE, Maizels RM (2011) Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11: 375–388. - PubMed
-
- Woolhouse ME, Taylor P, Matanhire D, Chandiwana SK (1991) Acquired immunity and epidemiology of Schistosoma haematobium. Nature 351: 757–759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

