Elephant endotheliotropic herpesvirus 5, a newly recognized elephant herpesvirus associated with clinical and subclinical infections in captive Asian elephants (Elephas maximus)
- PMID: 23505714
- PMCID: PMC3746547
- DOI: 10.1638/1042-7260-44.1.136
Elephant endotheliotropic herpesvirus 5, a newly recognized elephant herpesvirus associated with clinical and subclinical infections in captive Asian elephants (Elephas maximus)
Abstract
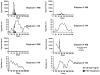
Elephant endotheliotropic herpesviruses (EEHVs) can cause acute hemorrhagic disease with high mortality rates in Asian elephants (Elephas maximus). Recently, a new EEHV type known as EEHV5 has been described, but its prevalence and clinical significance remain unknown. In this report, an outbreak of EEHV5 infection in a herd of captive Asian elephants in a zoo was characterized. In February 2011, a 42-yr-old wild-born female Asian elephant presented with bilaterally swollen temporal glands, oral mucosal hyperemia, vesicles on the tongue, and generalized lethargy. The elephant had a leukopenia and thrombocytopenia. She was treated with flunixin meglumine, famciclovir, and fluids. Clinical signs of illness resolved gradually over 2 wk, and the white blood cell count and platelets rebounded to higher-than-normal values. EEHV5 viremia was detectable starting 1 wk before presentation and peaked at the onset of clinical illness. EEHV5 shedding in trunk secretions peaked after viremia resolved and continued for more than 2 mo. EEHV5 trunk shedding from a female herd mate without any detectable viremia was detected prior to onset of clinical disease in the 42-yr-old elephant, indicating reactivation rather than primary infection in this elephant. Subsequent EEHV5 viremia and trunk shedding was documented in the other five elephants in the herd, who remained asymptomatic, except for 1 day of temporal gland swelling in an otherwise-healthy 1-yr-old calf. Unexpectedly, the two elephants most recently introduced into the herd 40 mo previously shed a distinctive EEHV5 strain from that seen in the other five elephants. This is the first report to document the kinetics of EEHV5 infection in captive Asian elephants and to provide evidence that this virus can cause illness in some animals.
Figures



References
-
- Cohen JI, Straus SE, Arvin AM. Varicellazoster virus replication, pathogenesis, and management. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2007. pp. 2773–2818.
-
- Fickel J, Richman LK, Montali R, Schaftenaar W, Goritz F, Hildebrandt TB, Pitra C. A variant of the endotheliotropic herpesvirus in Asian elephants (Elephas maximus) in European zoos. Vet Microbiol. 2001;82:103–109. - PubMed
-
- Garner MM, Helmick K, Ochsenreiter J, Richman LK, Latimer E, Wise AG, Maes RK, Kiupel M, Nordhausen RW, Zong JC, Hayward GS. Clinicopathologic features of fatal disease attributed to new variants of endotheliotropic herpesviruses in two Asian elephants (Elephas maximus) Vet Pathol. 2009;46:97–104. - PMC - PubMed
-
- Latimer E, Zong JC, Heaggans SY, Richman LK, Hayward GS. Detection and evaluation of novel herpesviruses in routine and pathological samples from Asian and African elephants: identification of two new probosciviruses (EEHV5 and EEHV6) and two new gammaherpesviruses (EGHV3B and EGHV5) Vet Microbiol. 2011;147:28–41. - PMC - PubMed
-
- Mocarski ES, Shenk T, Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2007. pp. 2701–2772.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

