Pathogenic mechanisms implicated in the intravascular coagulation in the lungs of BVDV-infected calves challenged with BHV-1
- PMID: 23506546
- PMCID: PMC3618313
- DOI: 10.1186/1297-9716-44-20
Pathogenic mechanisms implicated in the intravascular coagulation in the lungs of BVDV-infected calves challenged with BHV-1
Abstract
Resistance to respiratory disease in cattle requires host defense mechanisms that protect against pathogens which have evolved sophisticated strategies to evade them, including an altered function of pulmonary macrophages (MΦs) or the induction of inflammatory responses that cause lung injury and sepsis. The aim of this study was to clarify the mechanisms responsible for vascular changes occurring in the lungs of calves infected with bovine viral diarrhea virus (BVDV) and challenged later with bovine herpesvirus type 1 (BHV-1), evaluating the role of MΦs in the development of pathological lesions in this organ. For this purpose, pulmonary lesions were compared between co-infected calves and healthy animals inoculated only with BHV-1 through immunohistochemical (MAC387, TNFα, IL-1α, iNOS, COX-2 and Factor-VIII) and ultrastructural studies. Both groups of calves presented important vascular alterations produced by fibrin microthrombi and platelet aggregations within the blood vessels. These findings were earlier and more severe in the co-infected group, indicating that the concomitance of BVDV and BHV-1 in the lungs disrupts the pulmonary homeostasis by facilitating the establishment of an inflammatory and procoagulant environment modulated by inflammatory mediators released by pulmonary MΦs. In this regard, the co-infected calves, in spite of presenting a greater number of IMΦs than single-infected group, show a significant decrease in iNOS expression coinciding with the presence of more coagulation lesions. Moreover, animals pre-inoculated with BVDV displayed an alteration in the response of pro-inflammatory cytokines (TNFα and IL-1), which play a key role in activating the immune response, as well as in the local cell-mediated response.
Figures
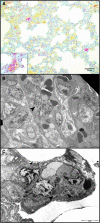

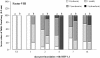
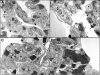




References
-
- Barrett DC. Bovine respiratory disease-A clinician’s perspective. Cattle Pract. 1998;6:251–255.
-
- Caldow G, Nettleton P. Pneumonia: identifying the causal agent. Cattle Pract. 2000;8:131–134.
-
- Griffin D. Economic impact associated with respiratory disease in beef cattle. Vet Clin North Am Food Anim Pract. 1997;13:367–377. - PubMed
-
- Fulton RW, Purdy CW, Confer AW, Saliki JT, Loan RW, Briggs RE, Burge LJ. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can J Vet Res. 2000;64:151–159. - PMC - PubMed
-
- Fulton RW, Blood KS, Panciera RJ, Payton ME, Ridpath JF, Confer AW, Saliki JT, Burge LT, Welsh RD, Johnson BJ, Reck A. Lung pathology and infectious agents in fatal feedlot pneumonias and relationship with mortality, disease onset, and treatments. J Vet Diagn Invest. 2009;21:464–477. doi: 10.1177/104063870902100407. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

