SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine
- PMID: 23506877
- PMCID: PMC3727660
- DOI: 10.1016/j.mam.2012.07.005
SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine
Abstract
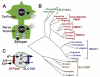
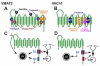
The exocytotic release of neurotransmitters requires active transport into synaptic vesicles and other types of secretory vesicles. Members of the SLC18 family perform this function for acetylcholine (SLC18A3, the vesicular acetylcholine transporter or VAChT) and monoamines such as dopamine and serotonin (SLC18A1 and 2, the vesicular monoamine transporters VMAT1 and 2, respectively). To date, no specific diseases have been attributed to a mutation in an SLC18 family member; however, polymorphisms in SLC18A1 and SLC18A2 may confer risk for some neuropsychiatric disorders. Additional members of this family include SLC18A4, expressed in insects, and SLC18B1, the function of which is not known. SLC18 is part of the Drug:H(+) Antiporter-1 Family (DHA1, TCID 2.A.1.2) within the Major Facilitator Superfamily (MFS, TCID 2.A.1).
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
References
-
- Brooks ES, Greer CL, Romero-Calderon R, Serway CN, Grygoruk A, Haimovitz JM, Nguyen BT, Najibi R, Tabone CJ, Steven de Belle J, Krantz DE. A putative vesicular transporter expressed in drosophila mushroom bodies that mediates sexual behavior may define a neurotransmitter system. Neuron. 2011;72:316–329. - PMC - PubMed
-
- Brunk I, Holtje M, von Jagow B, Winter S, Sternberg J, Blex C, Pahner I, Ahnert-Hilger G. Regulation of vesicular monoamine and glutamate transporters by vesicle-associated trimeric G proteins: new jobs for long-known signal transduction molecules. Handb. Exp. Pharmacol. 2006;175:305–325. - PubMed
-
- Chaudhry FA, Boulland JL, Jenstad M, Bredahl MK, Edwards RH. Pharmacology of neurotransmitter transport into secretory vesicles. Handb. Exp. Pharmacol. 2008;184:77–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

