The SLC37 family of phosphate-linked sugar phosphate antiporters
- PMID: 23506893
- PMCID: PMC3602828
- DOI: 10.1016/j.mam.2012.05.010
The SLC37 family of phosphate-linked sugar phosphate antiporters
Abstract
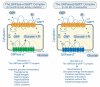
The SLC37 family consists of four sugar-phosphate exchangers, A1, A2, A3, and A4, which are anchored in the endoplasmic reticulum (ER) membrane. The best characterized family member is SLC37A4, better known as the glucose-6-phosphate (G6P) transporter (G6PT). SLC37A1, SLC37A2, and G6PT function as phosphate (Pi)-linked G6P antiporters catalyzing G6P:Pi and Pi:Pi exchanges. The activity of SLC37A3 is unknown. G6PT translocates G6P from the cytoplasm into the lumen of the ER where it couples with either glucose-6-phosphatase-α (G6Pase-α) or G6Pase-β to hydrolyze intraluminal G6P to glucose and Pi. The functional coupling of G6PT with G6Pase-α maintains interprandial glucose homeostasis and the functional coupling of G6PT with G6Pase-β maintains neutrophil energy homeostasis and functionality. A deficiency in G6PT causes glycogen storage disease type Ib, an autosomal recessive disorder characterized by impaired glucose homeostasis, neutropenia, and neutrophil dysfunction. Neither SLC37A1 nor SLC37A2 can functionally couple with G6Pase-α or G6Pase-β, and there are no known disease associations for them or SLC37A3. Since only G6PT matches the characteristics of the physiological ER G6P transporter involved in blood glucose homeostasis and neutrophil energy metabolism, the biological roles for the other SLC37 proteins remain to be determined.
Published by Elsevier Ltd.
Figures


References
-
- Adachi M, Shinkai M, Ohhama Y, Tachibana K, Kuratsuji T, Saji H, Maruya E. Improved neutrophil function in a glycogen storage disease type 1b patient after liver transplantation. Eur. J. Pediatr. 2004;163(4-5):202–206. - PubMed
-
- Angaroni CJ, Labrune P, Petit F, Sastre D, Capra AE, Dodelson de Kremer R, Argaraña CE. Glycogen storage disease type Ib without neutropenia generated by a novel splice-site mutation in the glucose-6-phosphate translocase gene. Mol. Genet. Metab. 2006;88(1):96–99. - PubMed
-
- Bartoloni L, Antonarakis SE. The human sugar-phosphate/phosphate exchanger family SLC37. Pflugers Arch. 2004;447(5):780–783. - PubMed
-
- Bartoloni L, Wattenhofer M, Kudoh J, Berry A, Shibuya K, Kawasaki K, Wang J, Asakawa S, Talior I, Bonne-Tamir B, Rossier C, Michaud J, McCabe ER, Minoshima S, Shimizu N, Scott HS, Antonarakis SE. Cloning and characterization of a putative human glycerol 3-phosphate permease gene (SLC37A1 or G3PP) on 21q22.3: mutation analysis in two candidate phenotypes, DFNB10 and a glycerol kinase deficiency. Genomics. 2000;70(2):190–200. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

