The effects of aging and Alzheimer's disease on cerebral cortical anatomy: specificity and differential relationships with cognition
- PMID: 23507382
- PMCID: PMC4098706
- DOI: 10.1016/j.neuroimage.2013.02.059
The effects of aging and Alzheimer's disease on cerebral cortical anatomy: specificity and differential relationships with cognition
Abstract
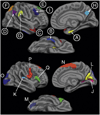
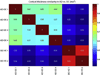
Although both normal aging and Alzheimer's disease (AD) are associated with regional cortical atrophy, few studies have directly compared the spatial patterns and magnitude of effects of these two processes. The extant literature has not addressed two important questions: 1) Is the pattern of age-related cortical atrophy different if cognitively intact elderly individuals with silent AD pathology are excluded? and 2) Does the age- or AD-related atrophy relate to cognitive function? Here we studied 142 young controls, 87 older controls, and 28 mild AD patients. In addition, we studied 35 older controls with neuroimaging data indicating the absence of brain amyloid. Whole-cortex analyses identified regions of interest (ROIs) of cortical atrophy in aging and in AD. Results showed that some regions are predominantly affected by age with relatively little additional atrophy in patients with AD, e.g., calcarine cortex; other regions are predominantly affected by AD with much less of an effect of age, e.g., medial temporal cortex. Finally, other regions are affected by both aging and AD, e.g., dorsolateral prefrontal cortex and inferior parietal lobule. Thus, the processes of aging and AD have both differential and partially overlapping effects on specific regions of the cerebral cortex. In particular, some frontoparietal regions are affected by both processes, most temporal lobe regions are affected much more prominently by AD than aging, while sensorimotor and some prefrontal regions are affected specifically by aging and minimally more by AD. Within normal older adults, atrophy in aging-specific cortical regions relates to cognitive performance, while in AD patients atrophy in AD-specific regions relates to cognitive performance. Further work is warranted to investigate the behavioral and clinical relevance of these findings in additional detail, as well as their histological basis; ROIs generated from the present study could be used strategically in such investigations.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



References
-
- Allen JS, Damasio H, Grabowski TJ. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am. Phys. Anthropol. 2002;118:341–358. - PubMed
-
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol. Aging. 2005;26:1245–1260. (discussion 1279–1282). - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb. Cortex. 1991;1:103–116. - PubMed
-
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42:1681–1688. - PubMed
Publication types
MeSH terms
Grants and funding
- U24 RR021382/RR/NCRR NIH HHS/United States
- P01-AG03991/AG/NIA NIH HHS/United States
- P41-RR14075/RR/NCRR NIH HHS/United States
- P50-AG05134/AG/NIA NIH HHS/United States
- R01 AG029411/AG/NIA NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- R01-AG29411/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- R21-AG29840/AG/NIA NIH HHS/United States
- P50-AG05681/AG/NIA NIH HHS/United States
- U24-RR021382/RR/NCRR NIH HHS/United States
- R21 AG029840/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

