A balance of form and function: planar polarity and development of the vestibular maculae
- PMID: 23507521
- PMCID: PMC3690145
- DOI: 10.1016/j.semcdb.2013.03.001
A balance of form and function: planar polarity and development of the vestibular maculae
Abstract
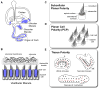
The mechanosensory hair cells of the inner ear have emerged as one of the primary models for studying the development of planar polarity in vertebrates. Planar polarity is the polarized organization of cells or cellular structures in the plane of an epithelium. For hair cells, planar polarity is manifest at the subcellular level in the polarized organization of the stereociliary bundle and at the cellular level in the coordinated orientation of stereociliary bundles between adjacent cells. This latter organization is commonly called Planar Cell Polarity and has been described in the greatest detail for auditory hair cells of the cochlea. A third level of planar polarity, referred to as tissue polarity, occurs in the utricular and saccular maculae; two inner ear sensory organs that use hair cells to detect linear acceleration and gravity. In the utricle and saccule hair cells are divided between two groups that have opposite stereociliary bundle polarities and, as a result, are able to detect movements in opposite directions. Thus vestibular hair cells are a unique model system for studying planar polarity because polarization develops at three different anatomical scales in the same sensory organ. Moreover the system has the potential to be used to dissect functional interactions between molecules regulating planar polarity at each of the three levels. Here the significance of planar polarity on vestibular system function will be discussed, and the molecular mechanisms associated with development of planar polarity at each anatomical level will be reviewed. Additional aspects of planar polarity that are unique to the vestibular maculae will also be introduced.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–58. - PubMed
-
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

