The Lipid Transfer Protein StarD7: Structure, Function, and Regulation
- PMID: 23507753
- PMCID: PMC3634439
- DOI: 10.3390/ijms14036170
The Lipid Transfer Protein StarD7: Structure, Function, and Regulation
Abstract
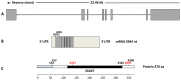
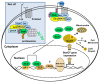
The steroidogenic acute regulatory (StAR) protein-related lipid transfer (START) domain proteins constitute a family of evolutionarily conserved and widely expressed proteins that have been implicated in lipid transport, metabolism, and signaling. The 15 well-characterized mammalian START domain-containing proteins are grouped into six subfamilies. The START domain containing 7 mRNA encodes StarD7, a member of the StarD2/phosphatidylcholine transfer protein (PCTP) subfamily, which was first identified as a gene overexpressed in a choriocarcinoma cell line. Recent studies show that the StarD7 protein facilitates the delivery of phosphatidylcholine to the mitochondria. This review summarizes the latest advances in StarD7 research, focusing on the structural and biochemical features, protein-lipid interactions, and mechanisms that regulate StarD7 expression. The implications of the role of StarD7 in cell proliferation, migration, and differentiation are also discussed.
Figures

Similar articles
-
StarD7 knockdown modulates ABCG2 expression, cell migration, proliferation, and differentiation of human choriocarcinoma JEG-3 cells.PLoS One. 2012;7(8):e44152. doi: 10.1371/journal.pone.0044152. Epub 2012 Aug 29. PLoS One. 2012. PMID: 22952907 Free PMC article.
-
StarD7 mediates the intracellular trafficking of phosphatidylcholine to mitochondria.J Biol Chem. 2010 Mar 5;285(10):7358-65. doi: 10.1074/jbc.M109.056960. Epub 2009 Dec 30. J Biol Chem. 2010. PMID: 20042613 Free PMC article.
-
StarD7 gene expression in trophoblast cells: contribution of SF-1 and Wnt-beta-catenin signaling.Mol Endocrinol. 2011 Aug;25(8):1364-75. doi: 10.1210/me.2010-0503. Epub 2011 May 26. Mol Endocrinol. 2011. PMID: 21622533 Free PMC article.
-
Give lipids a START: the StAR-related lipid transfer (START) domain in mammals.J Cell Sci. 2005 Jul 1;118(Pt 13):2791-801. doi: 10.1242/jcs.02485. J Cell Sci. 2005. PMID: 15976441 Review.
-
[START domain-containing proteins: a review of their role in lipid transport and exchange].Med Sci (Paris). 2009 Feb;25(2):181-91. doi: 10.1051/medsci/2009252181. Med Sci (Paris). 2009. PMID: 19239851 Review. French.
Cited by
-
Cis-perturbation of cancer drivers by the HTLV-1/BLV proviruses is an early determinant of leukemogenesis.Nat Commun. 2017 May 23;8:15264. doi: 10.1038/ncomms15264. Nat Commun. 2017. PMID: 28534499 Free PMC article.
-
Regulation and Essentiality of the StAR-related Lipid Transfer (START) Domain-containing Phospholipid Transfer Protein PFA0210c in Malaria Parasites.J Biol Chem. 2016 Nov 11;291(46):24280-24292. doi: 10.1074/jbc.M116.740506. Epub 2016 Oct 2. J Biol Chem. 2016. PMID: 27694132 Free PMC article.
-
A pre-vaccination immune metabolic interplay determines the protective antibody response to a dengue virus vaccine.Cell Rep. 2024 Jul 23;43(7):114370. doi: 10.1016/j.celrep.2024.114370. Epub 2024 Jun 18. Cell Rep. 2024. PMID: 38900640 Free PMC article.
-
StarD7 Protein Deficiency Adversely Affects the Phosphatidylcholine Composition, Respiratory Activity, and Cristae Structure of Mitochondria.J Biol Chem. 2016 Nov 25;291(48):24880-24891. doi: 10.1074/jbc.M116.736793. Epub 2016 Sep 30. J Biol Chem. 2016. PMID: 27694445 Free PMC article.
-
Sphingosine-1-Phosphate Lyase Deficient Cells as a Tool to Study Protein Lipid Interactions.PLoS One. 2016 Apr 21;11(4):e0153009. doi: 10.1371/journal.pone.0153009. eCollection 2016. PLoS One. 2016. PMID: 27100999 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

