The role of protein synthesis and digestive enzymes in acinar cell injury
- PMID: 23507798
- PMCID: PMC3902846
- DOI: 10.1038/nrgastro.2013.36
The role of protein synthesis and digestive enzymes in acinar cell injury
Abstract
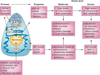
The exocrine pancreas is the organ with the highest level of protein synthesis in the adult--each day the pancreas produces litres of fluid filled with enzymes that are capable of breaking down nearly all organic substances. For optimal health, the pancreas must produce sufficient enzymes of the right character to match the dietary intake. Disruption of normal pancreatic function occurs primarily as a result of dysfunction of the acinar cells that produce these digestive enzymes, and can lead to acute or chronic diseases. For many years, the prevailing dogma has been that inappropriate intracellular activation of the digestive enzymes produced by acinar cells was the key to pancreatic inflammatory diseases, as digestive enzymes themselves are potentially harmful to the cells that secrete them. However, we now know that many stressors can affect pancreatic acinar cells, and that these stressors can independently trigger pancreatic pathology through various mechanisms. This Review focuses on protein synthesis and active digestive enzymes--two key stressors faced by the acinar cell that are likely to be the major drivers of pathology encountered in the pancreas.
Figures



References
-
- Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol. Rev. Camb. Philos. Soc. 1978;53:211–354. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62:10–29. - PubMed
-
- Weng N, Thomas DD, Groblewski GE. Pancreatic acinar cells express vesicle-associated membrane protein 2- and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. J. Biol. Chem. 2007;282:9635–9645. - PubMed
-
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

