Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3
- PMID: 23508103
- PMCID: PMC3647969
- DOI: 10.1128/MCB.01658-12
Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3
Abstract
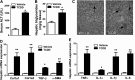
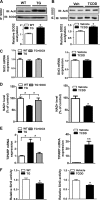
Nonalcoholic steatohepatitis (NASH) is a liver disorder that still demands improved treatment. Understanding the pathogenesis of NASH will help to develop novel approaches to prevent or treat this disease. In this study, we revealed a novel function of the aryl hydrocarbon receptor (AhR) in NASH. Transgenic or pharmacological activation of AhR heightened animal sensitivity to NASH induced by the methionine- and choline-deficient (MCD) diet, which was reasoned to be due to increased hepatic steatosis, production of reactive oxygen species (ROS), and lipid peroxidation. Mechanistically, the increased ROS production in AhR-activated mouse liver was likely a result of a lower superoxide dismutase 2 (SOD2) activity and compromised clearance of ROS. Activation of AhR induced tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly(ADP-ribose) polymerase (TiPARP) gene expression, depleted NAD(+), deactivated the mitochondrial sirtuin deacetylase 3 (Sirt3), increased SOD2 acetylation, and thereby decreased SOD2 activity. We also showed that Sirt3 ablation sensitized mice to NASH, whereas adenoviral overexpression of Sirt3 alleviated the NASH phenotype in AhR-transgenic mice. We conclude that activation of AhR sensitizes mice to NASH by facilitating both the "first hit" of steatosis and the "second hit" of oxidative stress. Our results suggest that the use of AhR antagonists might be a viable approach to prevent and treat NASH. Manipulation of the expression or activity of Sirt3 may also represent a novel approach to manage NASH.
Figures





Similar articles
-
CREBH alleviates mitochondrial oxidative stress through SIRT3 mediating deacetylation of MnSOD and suppression of Nlrp3 inflammasome in NASH.Free Radic Biol Med. 2022 Sep;190:28-41. doi: 10.1016/j.freeradbiomed.2022.07.018. Epub 2022 Aug 2. Free Radic Biol Med. 2022. PMID: 35926687
-
Aryl hydrocarbon receptor activation by dioxin targets phosphoenolpyruvate carboxykinase (PEPCK) for ADP-ribosylation via 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly(ADP-ribose) polymerase (TiPARP).J Biol Chem. 2013 Jul 26;288(30):21514-25. doi: 10.1074/jbc.M113.458067. Epub 2013 Jun 14. J Biol Chem. 2013. PMID: 23770670 Free PMC article.
-
Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor.Free Radic Biol Med. 2002 Nov 1;33(9):1268-78. doi: 10.1016/s0891-5849(02)01014-6. Free Radic Biol Med. 2002. PMID: 12398935
-
Functions of aryl hydrocarbon receptor (AHR) and CD38 in NAD metabolism and nonalcoholic steatohepatitis (NASH).Biochem Pharmacol. 2019 Nov;169:113620. doi: 10.1016/j.bcp.2019.08.022. Epub 2019 Aug 26. Biochem Pharmacol. 2019. PMID: 31465774 Review.
-
NAD+ precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms.Exp Neurol. 2020 Mar;325:113144. doi: 10.1016/j.expneurol.2019.113144. Epub 2019 Dec 16. Exp Neurol. 2020. PMID: 31837320 Free PMC article. Review.
Cited by
-
Constitutive Activation of the Human Aryl Hydrocarbon Receptor in Mice Promotes Hepatocarcinogenesis Independent of Its Coactivator Gadd45b.Toxicol Sci. 2019 Feb 1;167(2):581-592. doi: 10.1093/toxsci/kfy263. Toxicol Sci. 2019. PMID: 30346592 Free PMC article.
-
Aryl Hydrocarbon Receptor Plays Protective Roles against High Fat Diet (HFD)-induced Hepatic Steatosis and the Subsequent Lipotoxicity via Direct Transcriptional Regulation of Socs3 Gene Expression.J Biol Chem. 2016 Mar 25;291(13):7004-16. doi: 10.1074/jbc.M115.693655. Epub 2016 Feb 10. J Biol Chem. 2016. PMID: 26865635 Free PMC article.
-
Aryl hydrocarbon receptor (AHR) functions in infectious and sterile inflammation and NAD+-dependent metabolic adaptation.Arch Toxicol. 2021 Nov;95(11):3449-3458. doi: 10.1007/s00204-021-03134-9. Epub 2021 Sep 24. Arch Toxicol. 2021. PMID: 34559251 Free PMC article. Review.
-
Sirtuins and nonalcoholic fatty liver disease.World J Gastroenterol. 2016 Dec 14;22(46):10084-10092. doi: 10.3748/wjg.v22.i46.10084. World J Gastroenterol. 2016. PMID: 28028356 Free PMC article. Review.
-
The Correlation between Serum Zinc Level and Liver Histology in Non-Alcoholic Steatohepatitis.Iran J Pathol. 2019 Winter;14(1):17-25. doi: 10.30699/IJP.14.1.17. Epub 2018 Dec 27. Iran J Pathol. 2019. PMID: 31531097 Free PMC article.
References
-
- Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. 2008. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol. Med. 14:72–81 - PubMed
-
- Day CP, James OF. 1998. Steatohepatitis: a tale of two “hits”? Gastroenterology 114:842–845 - PubMed
-
- Rolo AP, Teodoro JS, Palmeira CM. 2012. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 52:59–69 - PubMed
-
- Koek GH, Liedorp PR, Bast A. 2011. The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta 412:1297–1305 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
