Determining target engagement in living systems
- PMID: 23508173
- PMCID: PMC4004587
- DOI: 10.1038/nchembio.1211
Determining target engagement in living systems
Abstract
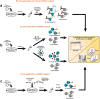
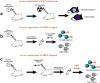
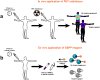
Chemical probes are critical tools for elucidating the biological functions of proteins and can lead to new medicines for treating disease. The pharmacological validation of protein function requires verification that chemical probes engage their intended targets in vivo. Here we discuss technologies, both established and emergent, for measuring target engagement in living systems and propose that determining this parameter should become standard practice for chemical probe and drug discovery programs.
Figures
References
-
- Grimwood S, Hartig PR. Target site occupancy: emerging generalizations from clinical and preclinical studies. Pharmacol Ther. 2009;122:281–301. - PubMed
-
- Wong DF, Tauscher J, Grunder G. The role of imaging in proof of concept for CNS drug discovery and development. Neuropsychopharmacology. 2009;34:187–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

