A biased activation theory of the cognitive and attentional modulation of emotion
- PMID: 23508210
- PMCID: PMC3600537
- DOI: 10.3389/fnhum.2013.00074
A biased activation theory of the cognitive and attentional modulation of emotion
Abstract
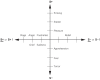
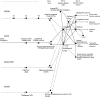
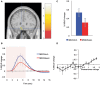
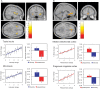
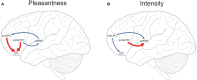
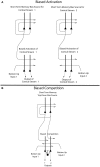
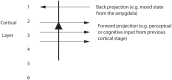
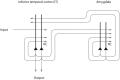
Cognition can influence emotion by biasing neural activity in the first cortical region in which the reward value and subjective pleasantness of stimuli is made explicit in the representation, the orbitofrontal cortex (OFC). The same effect occurs in a second cortical tier for emotion, the anterior cingulate cortex (ACC). Similar effects are found for selective attention, to for example the pleasantness vs. the intensity of stimuli, which modulates representations of reward value and affect in the orbitofrontal and anterior cingulate cortices. The mechanisms for the effects of cognition and attention on emotion are top-down biased competition and top-down biased activation. Affective and mood states can in turn influence memory and perception, by backprojected biasing influences. Emotion-related decision systems operate to choose between gene-specified rewards such as taste, touch, and beauty. Reasoning processes capable of planning ahead with multiple steps held in working memory in the explicit system can allow the gene-specified rewards not to be selected, or to be deferred. The stochastic, noisy, dynamics of decision-making systems in the brain may influence whether decisions are made by the selfish-gene-specified reward emotion system, or by the cognitive reasoning system that explicitly calculates reward values that are in the interests of the individual, the phenotype.
Keywords: cognition; decision-making; emotion; orbitofrontal cortex; planning; the noisy brain.
Figures
Similar articles
-
The orbitofrontal cortex and emotion in health and disease, including depression.Neuropsychologia. 2019 May;128:14-43. doi: 10.1016/j.neuropsychologia.2017.09.021. Epub 2017 Sep 24. Neuropsychologia. 2019. PMID: 28951164 Review.
-
Willed action, free will, and the stochastic neurodynamics of decision-making.Front Integr Neurosci. 2012 Sep 7;6:68. doi: 10.3389/fnint.2012.00068. eCollection 2012. Front Integr Neurosci. 2012. PMID: 22973205 Free PMC article.
-
The orbitofrontal cortex and beyond: from affect to decision-making.Prog Neurobiol. 2008 Nov;86(3):216-44. doi: 10.1016/j.pneurobio.2008.09.001. Epub 2008 Sep 7. Prog Neurobiol. 2008. PMID: 18824074 Review.
-
Attentional modulation of affective versus sensory processing: functional connectivity and a top-down biased activation theory of selective attention.J Neurophysiol. 2010 Sep;104(3):1649-60. doi: 10.1152/jn.00352.2010. Epub 2010 Jul 14. J Neurophysiol. 2010. PMID: 20631210 Clinical Trial.
-
Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala.Brain Struct Funct. 2023 Jun;228(5):1201-1257. doi: 10.1007/s00429-023-02644-9. Epub 2023 May 13. Brain Struct Funct. 2023. PMID: 37178232 Free PMC article. Review.
Cited by
-
A systematic review of reviews of neurocognitive functioning in eating disorders: The state-of-the-literature and future directions.Int J Eat Disord. 2018 Aug;51(8):798-821. doi: 10.1002/eat.22929. Epub 2018 Aug 13. Int J Eat Disord. 2018. PMID: 30102781 Free PMC article.
-
Altered cortical morphology in major depression disorder patients with suicidality.Psychoradiology. 2021 Mar 18;1(1):13-22. doi: 10.1093/psyrad/kkaa002. eCollection 2021 Mar. Psychoradiology. 2021. PMID: 38665310 Free PMC article.
-
The connections of neocortical pyramidal cells can implement the learning of new categories, attractor memory, and top-down recall and attention.Brain Struct Funct. 2021 Nov;226(8):2523-2536. doi: 10.1007/s00429-021-02347-z. Epub 2021 Aug 4. Brain Struct Funct. 2021. PMID: 34347165 Free PMC article.
-
Emotion, Motivation, Reasoning, and How Their Brain Systems Are Related.Brain Sci. 2025 May 16;15(5):507. doi: 10.3390/brainsci15050507. Brain Sci. 2025. PMID: 40426678 Free PMC article. Review.
-
Orbitofrontal cortex connectivity is associated with food reward and body weight in humans.Soc Cogn Affect Neurosci. 2023 Feb 6;18(1):nsab083. doi: 10.1093/scan/nsab083. Soc Cogn Affect Neurosci. 2023. PMID: 34189586 Free PMC article.
References
-
- Amaral D. G., Price J. L., Pitkanen A., Carmichael S. T. (1992). Anatomical organization of the primate amygdaloid complex, in The Amygdala, ed Aggleton J. P. (New York, NY: Wiley-Liss; ), 1–66
LinkOut - more resources
Full Text Sources
Other Literature Sources

