Interactions of zebrafish peptide YYb with the neuropeptide Y-family receptors Y4, Y7, Y8a, and Y8b
- PMID: 23508731
- PMCID: PMC3598007
- DOI: 10.3389/fnins.2013.00029
Interactions of zebrafish peptide YYb with the neuropeptide Y-family receptors Y4, Y7, Y8a, and Y8b
Abstract
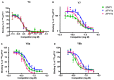
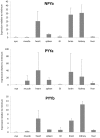
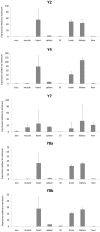
The neuropeptide Y (NPY) system influences numerous physiological functions including feeding behavior, endocrine regulation, and cardiovascular regulation. In jawed vertebrates it consists of 3-4 peptides and 4-7 receptors. Teleost fishes have unique duplicates of NPY and PYY as well as the Y8 receptor. In the zebrafish, the NPY system consists of the peptides NPYa, PYYa, and PYYb (NPYb appears to have been lost) and at least seven NPY receptors: Y1, Y2, Y2-2, Y4, Y7, Y8a, and Y8b. Previously PYYb binding has been reported for Y2 and Y2-2. To search for peptide-receptor preferences, we have investigated PYYb binding to four of the remaining receptors and compared with NPYa and PYYa. Taken together, the most striking observations are that PYYa displays reduced affinity for Y2 (3 nM) compared to the other peptides and receptors and that all three peptides have higher affinity for Y4 (0.028-0.034 nM) than for the other five receptors. The strongest peptide preference by any receptor selectivity is the one previously reported for PYYb by the Y2 receptor, as compared to NPY and PYYa. These affinity differences may be helpful to elucidate specific details of peptide-receptor interactions. Also, we have investigated the level of mRNA expression in different organs using qPCR. All peptides and receptors have higher expression in heart, kidney, and brain. These quantitative aspects on receptor affinities and mRNA distribution help provide a more complete picture of the NPY system.
Keywords: G-protein-coupled receptor; NPY; PYY; evolution; zebrafish.
Figures
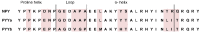
Similar articles
-
The Roles of Neuropeptide Y (Npy) and Peptide YY (Pyy) in Teleost Food Intake: A Mini Review.Life (Basel). 2021 Jun 10;11(6):547. doi: 10.3390/life11060547. Life (Basel). 2021. PMID: 34200824 Free PMC article. Review.
-
Duplication of neuropeptide Y and peptide YY in Nile tilapia Oreochromis niloticus and their roles in food intake regulation.Peptides. 2017 Feb;88:97-105. doi: 10.1016/j.peptides.2016.12.010. Epub 2016 Dec 14. Peptides. 2017. PMID: 27988351
-
Characterization of the neuropeptide Y system in the frog Silurana tropicalis (Pipidae): three peptides and six receptor subtypes.Gen Comp Endocrinol. 2012 Jul 1;177(3):322-31. doi: 10.1016/j.ygcen.2012.04.027. Epub 2012 May 4. Gen Comp Endocrinol. 2012. PMID: 22565163
-
Neuropeptide Y/peptide YY receptor Y2 duplicate in zebrafish with unique introns displays distinct peptide binding properties.Comp Biochem Physiol B Biochem Mol Biol. 2011 Dec;160(4):166-73. doi: 10.1016/j.cbpb.2011.08.001. Epub 2011 Aug 10. Comp Biochem Physiol B Biochem Mol Biol. 2011. PMID: 21855645
-
Molecular evolution of NPY receptor subtypes.Neuropeptides. 2004 Aug;38(4):141-51. doi: 10.1016/j.npep.2004.06.002. Neuropeptides. 2004. PMID: 15337367 Review.
Cited by
-
Perfluorooctanesulfonic Acid-Induced Toxicity on Zebrafish Embryos in the Presence or Absence of the Chorion.Environ Toxicol Chem. 2021 Mar;40(3):780-791. doi: 10.1002/etc.4899. Epub 2020 Dec 14. Environ Toxicol Chem. 2021. PMID: 33044770 Free PMC article.
-
Neuropeptide Y Regulates Sleep by Modulating Noradrenergic Signaling.Curr Biol. 2017 Dec 18;27(24):3796-3811.e5. doi: 10.1016/j.cub.2017.11.018. Epub 2017 Dec 7. Curr Biol. 2017. PMID: 29225025 Free PMC article.
-
The Roles of Neuropeptide Y (Npy) and Peptide YY (Pyy) in Teleost Food Intake: A Mini Review.Life (Basel). 2021 Jun 10;11(6):547. doi: 10.3390/life11060547. Life (Basel). 2021. PMID: 34200824 Free PMC article. Review.
-
Central neuropeptides as key modulators of astrocyte function in neurodegenerative and neuropsychiatric disorders.Psychopharmacology (Berl). 2025 Jun 19. doi: 10.1007/s00213-025-06840-9. Online ahead of print. Psychopharmacology (Berl). 2025. PMID: 40536717 Review.
-
Avian Neuropeptide Y: Beyond Feed Intake Regulation.Vet Sci. 2022 Apr 1;9(4):171. doi: 10.3390/vetsci9040171. Vet Sci. 2022. PMID: 35448669 Free PMC article. Review.
References
-
- Cerda-Reverter J. M., Larhammar D. (2000). Neuropeptide Y family of peptides: structure, anatomical expression, function, and molecular evolution. Biochem. Cell Biol. 78, 371–392 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

