Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism
- PMID: 23508959
- PMCID: PMC3642336
- DOI: 10.1074/jbc.M113.452953
Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism
Abstract
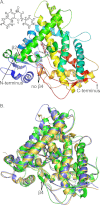
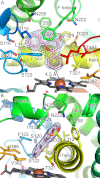
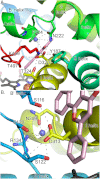
Cytochrome P450 (CYP) 1A1 is an extrahepatic monooxygenase involved in the metabolism of endogenous substrates and drugs, as well as the activation of certain toxins and environmental pollutants. CYP1A1 is particularly well known for its ability to biotransform polycyclic aromatic hydrocarbons, such as benzo[a]pyrene in tobacco smoke, into carcinogens. CYP1A1 possesses functional similarities and differences with human CYP1A2 and CYP1B1 enzymes, but the structural basis for this has been unclear. We determined a 2.6 Å structure of human CYP1A1 with the inhibitor α-naphthoflavone. α-Naphthoflavone binds within an enclosed active site, with the planar benzochromen-4-one core packed flat against the I helix that composes one wall of the active site, and the 2-phenyl substituent oriented toward the catalytic heme iron. Comparisons with previously determined structures of the related cytochrome P450 1A2 and 1B1 enzymes reveal distinct features among the active sites that may underlie the functional variability of these enzymes. Finally, docking studies probed the ability of CYP1A structures to assist in understanding their known in vitro interactions with several typical substrates and inhibitors.
Keywords: Cytochrome P450; Cytochrome P450 1A1; Docking; Drug Metabolism; Membrane Proteins; X-ray Crystallography; Xenobiotics.
Figures
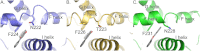


Similar articles
-
Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons.Chem Res Toxicol. 2006 Feb;19(2):288-94. doi: 10.1021/tx050291v. Chem Res Toxicol. 2006. PMID: 16485905
-
Theoretical investigation of differences in nitroreduction of aristolochic acid I by cytochromes P450 1A1, 1A2 and 1B1.Neuro Endocrinol Lett. 2012;33 Suppl 3:25-32. Neuro Endocrinol Lett. 2012. PMID: 23353840
-
Different mechanisms for inhibition of human cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic inhibitors.Chem Res Toxicol. 2007 Mar;20(3):489-96. doi: 10.1021/tx600299p. Epub 2007 Feb 10. Chem Res Toxicol. 2007. PMID: 17291012
-
Biological roles of cytochrome P450 1A1, 1A2, and 1B1 enzymes.Arch Pharm Res. 2021 Jan;44(1):63-83. doi: 10.1007/s12272-021-01306-w. Epub 2021 Jan 23. Arch Pharm Res. 2021. PMID: 33484438 Review.
-
Review of Ligand Specificity Factors for CYP1A Subfamily Enzymes from Molecular Modeling Studies Reported to-Date.Molecules. 2017 Jul 8;22(7):1143. doi: 10.3390/molecules22071143. Molecules. 2017. PMID: 28698457 Free PMC article. Review.
Cited by
-
Polycyclic aromatic hydrocarbons: from metabolism to lung cancer.Toxicol Sci. 2015 May;145(1):5-15. doi: 10.1093/toxsci/kfv040. Toxicol Sci. 2015. PMID: 25911656 Free PMC article. Review.
-
Substrate Selectivity of Coumarin Derivatives by Human CYP1 Enzymes: In Vitro Enzyme Kinetics and In Silico Modeling.ACS Omega. 2021 Apr 19;6(17):11286-11296. doi: 10.1021/acsomega.1c00123. eCollection 2021 May 4. ACS Omega. 2021. PMID: 34056284 Free PMC article.
-
Biological enrichment prediction of polychlorinated biphenyls and novel molecular design based on 3D-QSAR/HQSAR associated with molecule docking.Biosci Rep. 2019 May 17;39(5):BSR20180409. doi: 10.1042/BSR20180409. Print 2019 May 31. Biosci Rep. 2019. PMID: 31101726 Free PMC article.
-
A novel naphthalimide that selectively targets breast cancer via the arylhydrocarbon receptor pathway.Sci Rep. 2020 Aug 19;10(1):13978. doi: 10.1038/s41598-020-70597-8. Sci Rep. 2020. PMID: 32814815 Free PMC article.
-
Structure of an ancestral mammalian family 1B1 cytochrome P450 with increased thermostability.J Biol Chem. 2020 Apr 24;295(17):5640-5653. doi: 10.1074/jbc.RA119.010727. Epub 2020 Mar 10. J Biol Chem. 2020. PMID: 32156703 Free PMC article.
References
-
- Shimada T., Yun C. H., Yamazaki H., Gautier J. C., Beaune P. H., Guengerich F. P. (1992) Characterization of human lung microsomal cytochrome P-450 1A1 and its role in the oxidation of chemical carcinogens. Mol. Pharmacol. 41, 856–864 - PubMed
-
- Yengi L. G., Xiang Q., Pan J., Scatina J., Kao J., Ball S. E., Fruncillo R., Ferron G., Roland Wolf C. (2003) Quantitation of cytochrome P450 mRNA levels in human skin. Anal. Biochem. 316, 103–110 - PubMed
-
- van de Kerkhof E. G., de Graaf I. A., Ungell A. L., Groothuis G. M. (2008) Induction of metabolism and transport in human intestine. Validation of precision-cut slices as a tool to study induction of drug metabolism in human intestine in vitro. Drug Metab. Dispos. 36, 604–613 - PubMed
-
- Nishimura M., Yaguti H., Yoshitsugu H., Naito S., Satoh T. (2003) Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku zasshi 123, 369–375 - PubMed
-
- Hakkola J., Raunio H., Purkunen R., Pelkonen O., Saarikoski S., Cresteil T., Pasanen M. (1996) Detection of cytochrome P450 gene expression in human placenta in first trimester of pregnancy. Biochem. Pharmacol. 52, 379–383 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

