One identity or more for telomeres?
- PMID: 23509004
- PMCID: PMC3598436
- DOI: 10.3389/fonc.2013.00048
One identity or more for telomeres?
Abstract
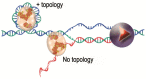
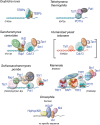
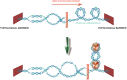
A major issue in telomere research is to understand how the integrity of chromosome ends is controlled. The fact that different types of nucleoprotein complexes have been described at the telomeres of different organisms raises the question of whether they have in common a structural identity that explains their role in chromosome protection. We will review here how telomeric nucleoprotein complexes are structured, comparing different organisms and trying to link these structures to telomere biology. It emerges that telomeres are formed by a complex and specific network of interactions between DNA, RNA, and proteins. The fact that these interactions and associated activities are reinforcing each other might help to guarantee the robustness of telomeric functions across the cell cycle and in the event of cellular perturbations. We will also discuss the recent notion that telomeres have evolved specific systems to overcome the DNA topological stress generated during their replication and transcription. This will lead to revisit the way we envisage the functioning of telomeric complexes since the regulation of topology is central to DNA stability, replication, recombination, and transcription as well as to chromosome higher-order organization.
Keywords: DNA topology; capping complexes; telomeres; telomeric chromatin organization.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

