Plasmodium coatneyi in rhesus macaques replicates the multisystemic dysfunction of severe malaria in humans
- PMID: 23509137
- PMCID: PMC3676004
- DOI: 10.1128/IAI.00027-13
Plasmodium coatneyi in rhesus macaques replicates the multisystemic dysfunction of severe malaria in humans
Abstract
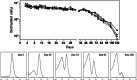
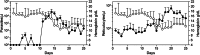
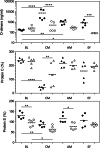
Severe malaria, a leading cause of mortality among children and nonimmune adults, is a multisystemic disorder characterized by complex clinical syndromes that are mechanistically poorly understood. The interplay of various parasite and host factors is critical in the pathophysiology of severe malaria. However, knowledge regarding the pathophysiological mechanisms and pathways leading to the multisystemic disorders of severe malaria in humans is limited. Here, we systematically investigate infections with Plasmodium coatneyi, a simian malaria parasite that closely mimics the biological characteristics of P. falciparum, and develop baseline data and protocols for studying erythrocyte turnover and severe malaria in greater depth. We show that rhesus macaques (Macaca mulatta) experimentally infected with P. coatneyi develop anemia, coagulopathy, and renal and metabolic dysfunction. The clinical course of acute infections required suppressive antimalaria chemotherapy, fluid support, and whole-blood transfusion, mimicking the standard of care for the management of severe malaria cases in humans. Subsequent infections in the same animals progressed with a mild illness in comparison, suggesting that immunity played a role in reducing the severity of the disease. Our results demonstrate that P. coatneyi infection in rhesus macaques can serve as a highly relevant model to investigate the physiological pathways and molecular mechanisms of malaria pathogenesis in naïve and immune individuals. Together with high-throughput postgenomic technologies, such investigations hold promise for the identification of new clinical interventions and adjunctive therapies.
Figures








References
-
- WHO 2011. World malaria report 2011. World Health Organization, Geneva, Switzerland
-
- Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I. 2008. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 5:e127 doi:10.1371/journal.pmed.0050127 - DOI - PMC - PubMed
-
- Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. 2008. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 5:e128 doi:10.1371/journal.pmed.0050128 - DOI - PMC - PubMed
-
- WHO 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl 1):1–90 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

