Bacterial flagella explore microscale hummocks and hollows to increase adhesion
- PMID: 23509269
- PMCID: PMC3619351
- DOI: 10.1073/pnas.1219662110
Bacterial flagella explore microscale hummocks and hollows to increase adhesion
Abstract
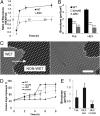
Biofilms, surface-bound communities of microbes, are economically and medically important due to their pathogenic and obstructive properties. Among the numerous strategies to prevent bacterial adhesion and subsequent biofilm formation, surface topography was recently proposed as a highly nonspecific method that does not rely on small-molecule antibacterial compounds, which promote resistance. Here, we provide a detailed investigation of how the introduction of submicrometer crevices to a surface affects attachment of Escherichia coli. These crevices reduce substrate surface area available to the cell body but increase overall surface area. We have found that, during the first 2 h, adhesion to topographic surfaces is significantly reduced compared with flat controls, but this behavior abruptly reverses to significantly increased adhesion at longer exposures. We show that this reversal coincides with bacterially induced wetting transitions and that flagellar filaments aid in adhesion to these wetted topographic surfaces. We demonstrate that flagella are able to reach into crevices, access additional surface area, and produce a dense, fibrous network. Mutants lacking flagella show comparatively reduced adhesion. By varying substrate crevice sizes, we determine the conditions under which having flagella is most advantageous for adhesion. These findings strongly indicate that, in addition to their role in swimming motility, flagella are involved in attachment and can furthermore act as structural elements, enabling bacteria to overcome unfavorable surface topographies. This work contributes insights for the future design of antifouling surfaces and for improved understanding of bacterial behavior in native, structured environments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bos R, van der Mei HC, Busscher HJ. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol Rev. 1999;23(2):179–230. - PubMed
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284(5418):1318–1322. - PubMed
-
- Park KD, et al. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials. 1998;19(7–9):851–859. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

