The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo
- PMID: 23509287
- PMCID: PMC3631677
- DOI: 10.1073/pnas.1221132110
The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo
Abstract
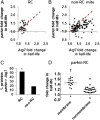
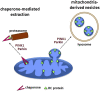
The accumulation of damaged mitochondria has been proposed as a key factor in aging and the pathogenesis of many common age-related diseases, including Parkinson disease (PD). Recently, in vitro studies of the PD-related proteins Parkin and PINK1 have found that these factors act in a common pathway to promote the selective autophagic degradation of damaged mitochondria (mitophagy). However, whether Parkin and PINK1 promote mitophagy under normal physiological conditions in vivo is unknown. To address this question, we used a proteomic approach in Drosophila to compare the rates of mitochondrial protein turnover in parkin mutants, PINK1 mutants, and control flies. We found that parkin null mutants showed a significant overall slowing of mitochondrial protein turnover, similar to but less severe than the slowing seen in autophagy-deficient Atg7 mutants, consistent with the model that Parkin acts upstream of Atg7 to promote mitophagy. By contrast, the turnover of many mitochondrial respiratory chain (RC) subunits showed greater impairment in parkin than Atg7 mutants, and RC turnover was also selectively impaired in PINK1 mutants. Our findings show that the PINK1-Parkin pathway promotes mitophagy in vivo and, unexpectedly, also promotes selective turnover of mitochondrial RC subunits. Failure to degrade damaged RC proteins could account for the RC deficits seen in many PD patients and may play an important role in PD pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Sequestration and autophagy of mitochondria do not cut proteins across the board.Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):6252-3. doi: 10.1073/pnas.1303921110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569251 Free PMC article. No abstract available.
References
-
- Karbowski M, Neutzner A. Neurodegeneration as a consequence of failed mitochondrial maintenance. Acta Neuropathol. 2012;123(2):157–171. - PubMed
-
- Horan MP, Pichaud N, Ballard JW. Review: Quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A Biol Sci Med Sci. 2012;67(10):1022–1035. - PubMed
-
- Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441(7097):1162–1166. - PubMed
-
- Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441(7097):1157–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

