CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells
- PMID: 23509298
- PMCID: PMC3619283
- DOI: 10.1073/pnas.1215438110
CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells
Abstract
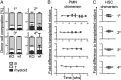
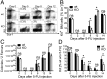
Pentatransmembrane glycoprotein prominin-1 (CD133) is expressed at the cell surface of multiple somatic stem cells, and it is widely used as a cell surface marker for the isolation and characterization of human hematopoietic stem cells (HSCs) and cancer stem cells. CD133 has been linked on a cell biological basis to stem cell-fate decisions in human HSCs and emerges as an important physiological regulator of stem cell maintenance and expansion. Its expression and physiological relevance in the murine hematopoietic system is nevertheless elusive. We show here that CD133 is expressed by bone marrow-resident murine HSCs and myeloid precursor cells with the developmental propensity to give rise to granulocytes and monocytes. However, CD133 is dispensable for the pool size and function of HSCs during steady-state hematopoiesis and after transplantation, demonstrating a substantial species difference between mouse and man. Blood cell numbers in the periphery are normal; however, CD133 appears to be a modifier for the development of growth-factor responsive myeloerythroid precursor cells in the bone marrow under steady state and mature red blood cells after hematopoietic stress. Taken together, these studies show that CD133 is not a critical regulator of hematopoietic stem cell function in mouse but that it modifies frequencies of growth-factor responsive hematopoietic progenitor cells during steady state and after myelotoxic stress in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Beckmann J, Scheitza S, Wernet P, Fischer JC, Giebel B. Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: Identification of asymmetrically segregating proteins. Blood. 2007;109(12):5494–5501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

