Development of a generic microfluidic device for simultaneous detection of antibodies and nucleic acids in oral fluids
- PMID: 23509739
- PMCID: PMC3586469
- DOI: 10.1155/2013/543294
Development of a generic microfluidic device for simultaneous detection of antibodies and nucleic acids in oral fluids
Abstract
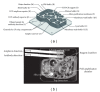
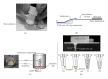
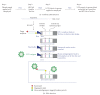
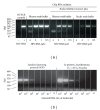
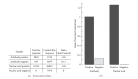
A prototype dual-path microfluidic device (Rheonix CARD) capable of performing simultaneously screening (antigen or antibody) and confirmatory (nucleic acid) detection of pathogens is described. The device fully integrates sample processing, antigen or antibody detection, and nucleic acid amplification and detection, demonstrating rapid and inexpensive "sample-to-result" diagnosis with performance comparable to benchtop analysis. For the chip design, a modular approach was followed allowing the optimization of individual steps in the sample processing process. This modular design provides great versatility accommodating different disease targets independently of the production method. In the detection module, a lateral flow (LF) protocol utilizing upconverting phosphor (UCP) reporters was employed. The nucleic acid (NA) module incorporates a generic microtube containing dry reagents. Lateral flow strips and PCR primers determine the target or disease that is diagnosed. Diagnosis of HIV infection was used as a model to investigate the simultaneous detection of both human antibodies against the virus and viral RNA. The serological result is available in less than 30 min, and the confirmation by RNA amplification takes another 60 min. This approach combines a core serological portable diagnostic with a nucleic acid-based confirmatory test.
Figures

Similar articles
-
An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids.Biomed Microdevices. 2010 Aug;12(4):705-19. doi: 10.1007/s10544-010-9423-4. Biomed Microdevices. 2010. PMID: 20401537 Free PMC article.
-
Integrated sample-to-detection chip for nucleic acid test assays.Biomed Microdevices. 2016 Jun;18(3):44. doi: 10.1007/s10544-016-0069-8. Biomed Microdevices. 2016. PMID: 27165104
-
Automatic and integrated detection of nucleic acid by using a dual-mode thermal controlled digital microfluidic chip.Anal Chim Acta. 2025 Jan 15;1334:343415. doi: 10.1016/j.aca.2024.343415. Epub 2024 Nov 13. Anal Chim Acta. 2025. PMID: 39638464
-
Recent advances in lab-on-a-chip technologies for viral diagnosis.Biosens Bioelectron. 2020 Apr 1;153:112041. doi: 10.1016/j.bios.2020.112041. Epub 2020 Jan 22. Biosens Bioelectron. 2020. PMID: 31999560 Free PMC article. Review.
-
Shaping up field-deployable nucleic acid testing using microfluidic paper-based analytical devices.Anal Bioanal Chem. 2019 Jul;411(19):4401-4414. doi: 10.1007/s00216-019-01595-7. Epub 2019 Feb 1. Anal Bioanal Chem. 2019. PMID: 30707267 Review.
Cited by
-
Simple Approaches to Minimally-Instrumented, Microfluidic-Based Point-of-Care Nucleic Acid Amplification Tests.Biosensors (Basel). 2018 Feb 26;8(1):17. doi: 10.3390/bios8010017. Biosensors (Basel). 2018. PMID: 29495424 Free PMC article. Review.
-
Improved assay to detect Plasmodium falciparum using an uninterrupted, semi-nested PCR and quantitative lateral flow analysis.Malar J. 2013 Feb 22;12:74. doi: 10.1186/1475-2875-12-74. Malar J. 2013. PMID: 23433252 Free PMC article.
-
Microfluidic Valves Made From Polymerized Polyethylene Glycol Diacrylate.Sens Actuators B Chem. 2014 Feb 1;191:10.1016/j.snb.2013.10.008. doi: 10.1016/j.snb.2013.10.008. Sens Actuators B Chem. 2014. PMID: 24357897 Free PMC article.
-
Field-evaluation of a new lateral flow assay for detection of cellular and humoral immunity against Mycobacterium leprae.PLoS Negl Trop Dis. 2014 May 8;8(5):e2845. doi: 10.1371/journal.pntd.0002845. eCollection 2014 May. PLoS Negl Trop Dis. 2014. PMID: 24810599 Free PMC article.
-
Microfluidic Device-Based Virus Detection and Quantification in Future Diagnostic Research: Lessons from the COVID-19 Pandemic.Biosensors (Basel). 2023 Oct 18;13(10):935. doi: 10.3390/bios13100935. Biosensors (Basel). 2023. PMID: 37887128 Free PMC article. Review.
References
-
- Hutchinson AB, Corbie-Smith G, Thomas SB, Mohanan S, Del Rio C. Understanding the patient’s perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Education and Prevention. 2004;16(2):101–114. - PubMed
-
- Hutchinson AB, Branson BM, Kim A, Farnham PG. A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS. 2006;20(12):1597–1604. - PubMed
-
- Malamud D, Abrams WR, Bau H, et al. Oral-based techniques for the diagnosis of infectious diseases. Journal of the California Dental Association. 2006;34(4):297–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

