A micro-silicon chip for in vivo cerebral imprint in monkey
- PMID: 23509975
- PMCID: PMC3605817
- DOI: 10.1021/cn300116g
A micro-silicon chip for in vivo cerebral imprint in monkey
Abstract
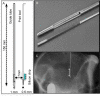
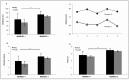
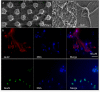
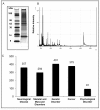
Access to cerebral tissue is essential to better understand the molecular mechanisms associated with neurodegenerative diseases. In this study, we present, for the first time, a new tool designed to obtain molecular and cellular cerebral imprints in the striatum of anesthetized monkeys. The imprint is obtained during a spatially controlled interaction of a chemically modified micro-silicon chip with the brain tissue. Scanning electron and immunofluorescence microscopies showed homogeneous capture of cerebral tissue. Nano-liquid chromatography-tandem mass spectrometry (nano-LC-MS/MS) analysis of proteins harvested on the chip allowed the identification of 1158 different species of proteins. The gene expression profiles of mRNA extracted from the imprint tool showed great similarity to those obtained via the gold standard approach, which is based on post-mortem sections of the same nucleus. Functional analysis of the harvested molecules confirmed the spatially controlled capture of striatal proteins implicated in dopaminergic regulation. Finally, the behavioral monitoring and histological results establish the safety of obtaining repeated cerebral imprints in striatal regions. These results demonstrate the ability of our imprint tool to explore the molecular content of deep brain regions in vivo. They open the way to the molecular exploration of brain in animal models of neurological diseases and will provide complementary information to current data mainly restricted to post-mortem samples.
Figures

Similar articles
-
A Laser Microdissection-Liquid Chromatography-Tandem Mass Spectrometry Workflow for Post-mortem Analysis of Brain Tissue.Methods Mol Biol. 2018;1723:371-383. doi: 10.1007/978-1-4939-7558-7_21. Methods Mol Biol. 2018. PMID: 29344872
-
Protocol for the Analysis of Laser Capture Microdissected Fresh-Frozen Tissue Homogenates by Silver-Stained 1D SDS-PAGE.Methods Mol Biol. 2018;1723:95-110. doi: 10.1007/978-1-4939-7558-7_4. Methods Mol Biol. 2018. PMID: 29344855
-
A silicon based implantable microelectrode array for electrophysiological and dopamine recording from cortex to striatum in the non-human primate brain.Biosens Bioelectron. 2016 Nov 15;85:53-61. doi: 10.1016/j.bios.2016.04.087. Epub 2016 Apr 27. Biosens Bioelectron. 2016. PMID: 27155116
-
Use of monolithic supports in proteomics technology.J Chromatogr A. 2007 Mar 9;1144(1):2-13. doi: 10.1016/j.chroma.2006.11.082. Epub 2006 Dec 15. J Chromatogr A. 2007. PMID: 17174320 Review.
-
Reaching for the deep proteome: recent nano liquid chromatography coupled with tandem mass spectrometry-based studies on the deep proteome.Arch Pharm Res. 2012 Nov;35(11):1861-70. doi: 10.1007/s12272-012-1102-y. Epub 2012 Dec 4. Arch Pharm Res. 2012. PMID: 23212627 Review.
References
-
- Moore D. J.; West A. B.; Dawson V. L.; Dawson T. M. (2005) Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 28, 57–87. - PubMed
-
- Duke D. C.; Moran L. B.; Kalaitzakis M. E.; Deprez M.; Dexter D. T.; Pearce R. K.; Graeber M. B. (2006) Transcriptome analysis reveals link between proteasomal and mitochondrial pathways in Parkinson’s disease. Neurogenetics 7, 139–148. - PubMed
-
- Moran L. B.; Duke D. C.; Deprez M.; Dexter D. T.; Pearce R. K.; Graeber M. B. (2006) Whole genome expression profiling of the medial and lateral substantia nigra in Parkinson’s disease. Neurogenetics 7, 1–11. - PubMed
-
- Mandel S.; Grunblatt E.; Riederer P.; Amariglio N.; Jacob-Hirsch J.; Rechavi G.; Youdim M. B. (2005) Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann. N.Y. Acad. Sci. 1053, 356–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

