Influence of the physiochemical properties of superparamagnetic iron oxide nanoparticles on amyloid β protein fibrillation in solution
- PMID: 23509983
- PMCID: PMC3605812
- DOI: 10.1021/cn300196n
Influence of the physiochemical properties of superparamagnetic iron oxide nanoparticles on amyloid β protein fibrillation in solution
Abstract
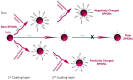
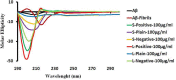
Superparamagnetic iron oxide nanoparticles (SPIONs) are recognized as promising nanodiagnostic materials due to their biocompatibility, unique magnetic properties, and their application as multimodal contrast agents. As coated SPIONs have potential use in the diagnosis and treatment of various brain diseases such as Alzheimer's, a comprehensive understanding of their interactions with Aβ and other amyloidogenic proteins is essential prior to their clinical application. Here we demonstrate the effect of thickness and surface charge of the coating layer of SPIONs on the kinetics of fibrillation of Aβ in aqueous solution. A size and surface area dependent "dual" effect on Aβ fibrillation was observed. While lower concentrations of SPIONs inhibited fibrillation, higher concentrations increased the rate of Aβ fibrillation. With respect to coating charge, it is evident that the positively charged SPIONs are capable of promoting fibrillation at significantly lower particle concentrations compared with negatively charged or uncharged SPIONs. This suggests that in addition to the presence of particles, which affect the concentration of monomeric protein in solution (and thereby the nucleation time), there are also effects of binding on the protein conformation.
Figures




References
-
- Cleary J. P.; Walsh D. M.; Hofmeister J. J.; Shankar G. M.; Kuskowski M. A.; Selkoe D. J.; Ashe K. H. (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 8, 79–84. - PubMed
-
- Walsh D. M.; Klyubin I.; Shankar G. M.; Townsend M.; Fadeeva J. V.; Betts V.; Podlisny M. B.; Cleary J. P.; Ashe K. H.; Rowan M. J.; Selkoe D. J. (2005) The role of cell-derived oligomers of abeta in alzheimer‘s disease and avenues for therapeutic intervention. Biochem. Soc. Trans. 33, 1087–1090. - PubMed
-
- Selkoe D. J. (2002) Alzheimer s disease is a synaptic failure. Science 298, 789–791. - PubMed
-
- Lambert M. P.; Barlow A. K.; Chromy B. A.; Edwards C.; Freed R.; Liosatos M.; Morgan T. E.; Rozovsky I.; Trommer B.; Viola K. L.; Wals P.; Zhang C.; Finch C. E.; Krafft G. A.; Klien W. L. (1998) Diffusible, nonfibrillar ligands derived from Ab1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453. - PMC - PubMed
-
- Walsh D.; Klyubin I.; Fadeeva J. V.; Cullen W. K.; Anwyl R.; Wolfe M. S.; Rowan M. J.; Selkoe D. J. (2002) Naturally secreted oligomers of amyloid b protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

