Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells
- PMID: 23510015
- PMCID: PMC7657167
- DOI: 10.1111/cas.12154
Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells
Abstract
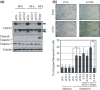
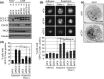
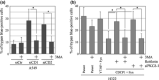
CUB (C1r/C1s, urchin embryonic growth factor, BMP1) domain-containing protein 1 (CDCP1) has been implicated in promoting metastasis of cancer cells through several mechanisms, including the inhibition of anoikis, which is cell death triggered by the loss of extracellular matrix interactions. However, the mechanism inhibiting cell death regulated by CDCP1 remains elusive. Inhibition of CDCP1 expression using small interfering RNA (siRNA) induced the cell death of suspended cancer cells without cleaving caspase-3, a marker of apoptosis; cell death was not inhibited by a general caspase inhibitor, suggesting that the loss of CDCP1 induces caspase-independent cell death. In contrast, knockdown of CDCP1 as well as protein kinase Cδ (PKCδ), a downstream effector of CDCP1, in a suspension culture of lung cancer cells resulted in marked induction of membranous microtubule-associated protein 1 light chain 3 (LC3)-II protein, a hallmark of autophagy, and caused the formation of an autophagosome structure visualized using green fluorescent protein-tagged LC3-II. Expression and phosphorylation of exogenous CDCP1 by Fyn kinase reduced the formation of autophagosomes and inhibited phosphorylation of CDCP1 by PP2, a Src kinase inhibitor or inhibited PKCδ by rottlerin, stimulating autophagosome formation. Moreover, death of suspended lung cancer cells induced by CDCP1 siRNA or by PKCδ siRNA was reduced by the autophagy inhibitor 3-methyladenine. These results indicate that CDCP1-PKCδ signaling plays a critical role in inhibiting autophagy, which is responsible for anoikis resistance of lung cancer cells.
© 2013 Japanese Cancer Association.
Figures

References
-
- Hooper JD, Zijlstra A, Aimes RT et al Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene 2003; 22: 1783–94. - PubMed
-
- Scherl‐Mostageer M, Sommergruber W, Abseher R, Hauptmann R, Ambros P, Schweifer N. Identification of a novel gene, CDCP1, overexpressed in human colorectal cancer. Oncogene 2001; 20: 4402–8. - PubMed
-
- Brown TA, Yang TM, Zaitsevskaia T et al Adhesion or plasmin regulates tyrosine phosphorylation of a novel membrane glycoprotein p80/gp140/CUB domain‐containing protein 1 in epithelia. J Biol Chem 2004; 279: 14772–83. - PubMed
-
- Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCδ is a phosphotyrosine binding domain. Cell 2005; 121: 271–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

