Gaucher's disease and cancer: a sphingolipid perspective
- PMID: 23510065
- PMCID: PMC3604879
- DOI: 10.1615/critrevoncog.2013005814
Gaucher's disease and cancer: a sphingolipid perspective
Abstract
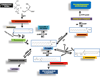
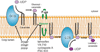
Gaucher's disease is a sphingolipidosis characterized by a specific deficiency in an acidic glucocerebrosidase, which results in aberrant accumulation of glucosylceramide primarily within the lysosome. Gaucher's disease has been correlated with cases of myeloma, leukemia, glioblastoma, lung cancer, and hepatocellular carcinoma, although the reasons for the correlation are currently being debated. Some suggest that the effects of Gaucher's disease may be linked to cancer, while others implicate the therapies used to treat Gaucher's disease. This debate is not entirely surprising, as the speculations linking Gaucher's disease with cancer fail to address the roles of ceramide and glucosylceramide in cancer biology. In this review, we will discuss, in the context of cancer biology, ceramide metabolism to glucosylceramide, the roles of glucosylceramide in multidrug-resistance, and the role of ceramide as an anticancer lipid. This review should reveal that it is most practical to associate elevated glucosylceramide, which accompanies Gaucher's disease, with the progression of cancer. Furthermore, this review proposes that the therapies used to treat Gaucher's disease, which augment ceramide accumulation, are likely not linked to correlations with cancer.
Conflict of interest statement
Penn State research foundation has licensed ceramide-based drug delivery systems to Keystone Nano, Inc. and Mark Kester is the Chief Medical officer of Keystone Nano.
Figures

References
-
- Barth BM, Cabot MC, Kester M. Ceramide-Based Therapeutics for the Treatment of Cancer. Anticancer Agents Med Chem. 2011;11(9):911–919. - PubMed
-
- Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther. 2011;11(2):138–149. - PubMed
-
- Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ. 2007;31(1):5–16. - PubMed
-
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–1859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

