Early versus deferred androgen suppression therapy for patients with lymph node-positive prostate cancer after local therapy with curative intent: a systematic review
- PMID: 23510155
- PMCID: PMC3621662
- DOI: 10.1186/1471-2407-13-131
Early versus deferred androgen suppression therapy for patients with lymph node-positive prostate cancer after local therapy with curative intent: a systematic review
Abstract
Background: There is currently no consensus regarding the optimal timing for androgen suppression therapy in patients with prostate cancer that have undergone local therapy with curative intent but are proven to have node-positive disease without signs of distant metastases at the time of local therapy. The objective of this systematic review was to determine the benefits and harms of early (at the time of local therapy) versus deferred (at the time of clinical disease progression) androgen suppression therapy for patients with node-positive prostate cancer after local therapy.
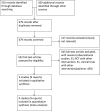
Methods: The protocol was registered prospectively (CRD42011001221; http://www.crd.york.ac.uk/PROSPERO). We searched the MEDLINE, EMBASE, and CENTRAL databases, as well as reference lists, the abstracts of three major conferences, and three trial registers, to identify randomized controlled trials (search update 04/08/2012). Two authors independently screened the identified articles, assessed trial quality, and extracted data.
Results: Four studies including 398 patients were identified for inclusion. Early androgen suppression therapy lead to a significant decrease in overall mortality (HR 0.62, 95% CI 0.46-0.84), cancer-specific mortality (HR 0.34, 95% CI 0.18-0.64), and clinical progression at 3 or 9 years (RR 0.29, 95% CI 0.16-0.52 at 3 years and RR 0.49, 95% CI 0.36-0.67 at 9 years). One study showed an increase of adverse effects with early androgen suppression therapy. All trials had substantial methodological limitations.
Conclusions: The data available suggest an improvement in survival and delayed disease progression but increased adverse events for patients with node-positive prostate cancer after local therapy treated with early androgen suppression therapy versus deferred androgen suppression therapy. However, quality of data is low. Randomized controlled trials with blinding of outcome assessment, planned to determine the timing of androgen suppression therapy in node-positive prostate cancer using modern diagnostic imaging modalities, biochemical testing, and standardized follow-up schedules should be conducted to confirm these findings.
Figures

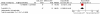


References
-
- Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, Pan L, Anderson RN, Fulton JE, Kohler BA. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–2366. doi: 10.1002/cncr.27514. - DOI - PMC - PubMed
-
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. - DOI - PubMed
-
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;167(2 Pt 2):948–951. discussion 952. - PubMed
-
- Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Schmid HP, Van der Kwast T, Wiegel T, Zattoni F. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59(4):572–583. doi: 10.1016/j.eururo.2011.01.025. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

