Redox-activated manganese-based MR contrast agent
- PMID: 23510406
- PMCID: PMC3622546
- DOI: 10.1021/ja312610j
Redox-activated manganese-based MR contrast agent
Abstract
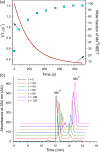
Here we report a simple Mn coordination complex with utility as a redox-sensitive MR probe. The HBET ligand stabilizes both the Mn(2+) and Mn(3+) oxidation states. In the presence of glutathione (GSH), low relaxivity Mn(III)-HBET is converted to high relaxivity Mn(II)-HBET with a 3-fold increase in relaxivity, and concomitant increase in MR signal. Alternately, hydrogen peroxide can convert Mn(II)-HBET to Mn(III)-HBET with a reduction in MR signal.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

