Hfq restructures RNA-IN and RNA-OUT and facilitates antisense pairing in the Tn10/IS10 system
- PMID: 23510801
- PMCID: PMC3677282
- DOI: 10.1261/rna.037747.112
Hfq restructures RNA-IN and RNA-OUT and facilitates antisense pairing in the Tn10/IS10 system
Abstract
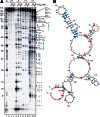
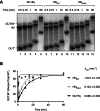
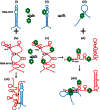
Hfq functions in post-transcriptional gene regulation in a wide range of bacteria, usually by promoting base-pairing of mRNAs and trans-encoded sRNAs that share partial sequence complementarity. It is less clear if Hfq is required for pairing of cis-encoded RNAs (i.e., antisense RNAs) with their target mRNAs. In the current work, we have characterized the interactions between Escherichia coli Hfq and the components of the Tn10/IS10 antisense system, RNA-IN and RNA-OUT. We show that Hfq interacts with RNA-OUT through its proximal RNA-binding surface, as is typical for Hfq and trans-encoded sRNAs. In contrast, RNA-IN binds both proximal and distal RNA-binding surfaces in Hfq with a higher affinity for the latter, as is typical for mRNA interactions in canonical sRNA-mRNA pairs. Importantly, an amino acid substitution in Hfq that interferes with RNA binding to the proximal site negatively impacts RNA-IN:OUT pairing in vitro and suppresses the ability of Hfq to negatively regulate IS10 transposition in vivo. We also show that Hfq binding to RNA-IN and RNA-OUT alters secondary structure elements in both of these RNAs and speculate that this could be important in how Hfq facilitates RNA-IN:OUT pairing. Based on the results presented here, we suggest that Hfq could be involved in regulating RNA pairing in other antisense systems, including systems encoded by other transposable elements.
Figures







References
-
- Altuvia S, Weinstein-Fischer D, Zhang A, Postow L, Storz G 1997. A small, stable RNA induced by oxidative stress: Role as a pleiotropic regulator and antimutator. Cell 90: 43–53 - PubMed
-
- Arini A, Keller MP, Arber W 1997. An antisense RNA in IS30 regulates the translational expression of the transposase. Biol Chem 378: 1421–1431 - PubMed
-
- Bolivar F, Backman K 1979. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol 68: 245–267 - PubMed
-
- Brennan RG, Link TM 2007. Hfq structure, function and ligand binding. Curr Opin Microbiol 10: 125–133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
