Engineering the human pluripotent stem cell microenvironment to direct cell fate
- PMID: 23510904
- PMCID: PMC3758782
- DOI: 10.1016/j.biotechadv.2013.03.002
Engineering the human pluripotent stem cell microenvironment to direct cell fate
Abstract
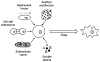
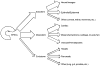
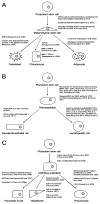
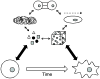
Human pluripotent stem cells (hPSCs), including both embryonic stem cells and induced pluripotent stem cells, offer a potential cell source for research, drug screening, and regenerative medicine applications due to their unique ability to self-renew or differentiate to any somatic cell type. Before the full potential of hPSCs can be realized, robust protocols must be developed to direct their fate. Cell fate decisions are based on components of the surrounding microenvironment, including soluble factors, substrate or extracellular matrix, cell-cell interactions, mechanical forces, and 2D or 3D architecture. Depending on their spatio-temporal context, these components can signal hPSCs to either self-renew or differentiate to cell types of the ectoderm, mesoderm, or endoderm. Researchers working at the interface of engineering and biology have identified various factors which can affect hPSC fate, often based on lessons from embryonic development, and they have utilized this information to design in vitro niches which can reproducibly direct hPSC fate. This review highlights culture systems that have been engineered to promote self-renewal or differentiation of hPSCs, with a focus on studies that have elucidated the contributions of specific microenvironmental cues in the context of those culture systems. We propose the use of microsystem technologies for high-throughput screening of spatial-temporal presentation of cues, as this has been demonstrated to be a powerful approach for differentiating hPSCs to desired cell types.
Keywords: Cell culture engineering; Differentiation; Ectoderm; Embryonic stem cells; Endoderm; Induced pluripotent stem cells; Mesoderm; Microenvironment; Niche; Self-renewal.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Human Pluripotent Stem Cell Mechanobiology: Manipulating the Biophysical Microenvironment for Regenerative Medicine and Tissue Engineering Applications.Stem Cells. 2015 Nov;33(11):3187-96. doi: 10.1002/stem.2105. Epub 2015 Jul 29. Stem Cells. 2015. PMID: 26189759 Review.
-
Stencil Micropatterning for Spatial Control of Human Pluripotent Stem Cell Fate Heterogeneity.Methods Mol Biol. 2016;1516:171-181. doi: 10.1007/7651_2016_325. Methods Mol Biol. 2016. PMID: 27032943
-
Mechanobiology of human pluripotent stem cells.Tissue Eng Part B Rev. 2013 Oct;19(5):420-30. doi: 10.1089/ten.TEB.2012.0641. Epub 2013 Apr 26. Tissue Eng Part B Rev. 2013. PMID: 23472616 Free PMC article. Review.
-
Glutamine-dependent signaling controls pluripotent stem cell fate.Dev Cell. 2022 Mar 14;57(5):610-623.e8. doi: 10.1016/j.devcel.2022.02.003. Epub 2022 Feb 24. Dev Cell. 2022. PMID: 35216682 Free PMC article.
-
Soft microenvironments promote the early neurogenic differentiation but not self-renewal of human pluripotent stem cells.Integr Biol (Camb). 2012 Sep;4(9):1049-58. doi: 10.1039/c2ib20083j. Epub 2012 Aug 2. Integr Biol (Camb). 2012. PMID: 22854634 Free PMC article.
Cited by
-
Nanoscale microenvironment engineering for expanding human hair follicle stem cell and revealing their plasticity.J Nanobiotechnology. 2021 Mar 31;19(1):94. doi: 10.1186/s12951-021-00840-5. J Nanobiotechnology. 2021. PMID: 33789665 Free PMC article.
-
On human pluripotent stem cell control: The rise of 3D bioengineering and mechanobiology.Biomaterials. 2015 Jun;52:26-43. doi: 10.1016/j.biomaterials.2015.01.078. Epub 2015 Feb 21. Biomaterials. 2015. PMID: 25818411 Free PMC article. Review.
-
Prediction of Human Induced Pluripotent Stem Cell Cardiac Differentiation Outcome by Multifactorial Process Modeling.Front Bioeng Biotechnol. 2020 Jul 23;8:851. doi: 10.3389/fbioe.2020.00851. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32793579 Free PMC article.
-
Microenvironmental regulation of telomerase isoforms in human embryonic stem cells.Stem Cells Dev. 2014 Sep 1;23(17):2046-66. doi: 10.1089/scd.2013.0373. Epub 2014 Jun 17. Stem Cells Dev. 2014. PMID: 24749509 Free PMC article.
-
Liquid Crystalline Hydroxyapatite Nanorods Orchestrate Hierarchical Bone-Like Mineralization.Small. 2024 Dec;20(52):e2310024. doi: 10.1002/smll.202310024. Epub 2024 Aug 23. Small. 2024. PMID: 39177175 Free PMC article.
References
-
- Aberdam E, Barak E, Rouleau M, de LaForest S, Berrih-Aknin S, Suter D, et al. A pure population of ectodermal cells derived from human embryonic stem cells. Stem Cells. 2008;26:440–4. - PubMed
-
- Agarwal S, Holton K, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–27. - PubMed
-
- Ahmad S, Stewart R, Yung S, Kolli S, Armstrong L, Stojkovic M, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145–55. - PubMed
-
- Ali N, Edgar A, Samadikuchaksaraei A, Timson C, Romanska H, Polak J, et al. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Engineering. 2002;8:541–50. - PubMed
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

