An improbable monometallic cluster entrapped in a popular fullerene cage: YCN@C(s)(6)-C82
- PMID: 23512079
- PMCID: PMC3601605
- DOI: 10.1038/srep01487
An improbable monometallic cluster entrapped in a popular fullerene cage: YCN@C(s)(6)-C82
Abstract
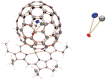
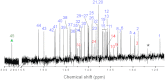
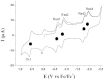
Since the first proposal that fullerenes are capable of hosting atoms, ions, or clusters by the late Smalley in 1985, tremendous examples of endohedral metallofullerenes (EMFs) have been reported. Breaking the dogma that monometallofullerenes (mono-EMFs) always exist in the form of M@C2n while clusterfullerenes always require multiple (two to four) metal cations to stabilize a cluster that is unstable as a single moiety, here we show an unprecedented monometallic endohedral clusterfullerene entrapping an yttrium cyanide cluster inside a popular C82 cage--YCN@C(s)(6)-C82. X-ray crystallography and (13)C NMR characterization unambiguously determine the cage symmetry and the endohedal cyanide structure, unexpectedly revealing that the entrapped YCN cluster is triangular. The unprecedented monometallic clusterfullerene structure unveiled by YCN@C(s)(6)-C82 opens up a new avenue for stabilizing a cluster by a single metal cation within a carbon cage, and will surely stimulate further studies on the stability and formation mechanism of EMFs.
Figures
Similar articles
-
Triangular Monometallic Cyanide Cluster Entrapped in Carbon Cage with Geometry-Dependent Molecular Magnetism.J Am Chem Soc. 2016 Nov 9;138(44):14764-14771. doi: 10.1021/jacs.6b09329. Epub 2016 Oct 31. J Am Chem Soc. 2016. PMID: 27755875 Free PMC article.
-
Fullerenes as Nanocontainers That Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity.Acc Chem Res. 2019 Jul 16;52(7):1824-1833. doi: 10.1021/acs.accounts.9b00229. Epub 2019 Jul 1. Acc Chem Res. 2019. PMID: 31260256
-
The inner-induced effects of YCN in C76 on the structures and nonlinear optical properties.J Mol Model. 2016 Aug;22(8):174. doi: 10.1007/s00894-016-3040-y. Epub 2016 Jul 6. J Mol Model. 2016. PMID: 27383610
-
Endohedral Metallofullerenes: New Structures and Unseen Phenomena.Chemistry. 2020 May 7;26(26):5748-5757. doi: 10.1002/chem.201905306. Epub 2020 Mar 13. Chemistry. 2020. PMID: 31886563 Review.
-
Gd@C82 Fullerenol.2008 Oct 31 [updated 2008 Dec 1]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Oct 31 [updated 2008 Dec 1]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641737 Free Books & Documents. Review.
Cited by
-
Methane as a Selectivity Booster in the Arc-Discharge Synthesis of Endohedral Fullerenes: Selective Synthesis of the Single-Molecule Magnet Dy2TiC@C80 and Its Congener Dy2TiC2@C80.Angew Chem Int Ed Engl. 2015 Nov 2;54(45):13411-5. doi: 10.1002/anie.201505870. Epub 2015 Sep 9. Angew Chem Int Ed Engl. 2015. PMID: 26350440 Free PMC article.
-
Endohedral metal-nitride cluster ordering in metallofullerene-NiII(OEP) complexes and crystals: a theoretical study.Phys Chem Chem Phys. 2019 Apr 17;21(16):8197-8200. doi: 10.1039/c9cp00634f. Phys Chem Chem Phys. 2019. PMID: 30816387 Free PMC article.
-
Mononuclear Clusterfullerene Single-Molecule Magnet Containing Strained Fused-Pentagons Stabilized by a Nearly Linear Metal Cyanide Cluster.Angew Chem Int Ed Engl. 2017 Feb 6;56(7):1830-1834. doi: 10.1002/anie.201611345. Epub 2017 Jan 12. Angew Chem Int Ed Engl. 2017. PMID: 28079303 Free PMC article.
-
Trapping an unprecedented Ti3C3 unit inside the icosahedral C80 fullerene: a crystallographic survey.Chem Sci. 2019 Oct 14;10(47):10925-10930. doi: 10.1039/c9sc04315b. eCollection 2019 Dec 21. Chem Sci. 2019. PMID: 32190248 Free PMC article.
-
Single Molecule Magnetism with Strong Magnetic Anisotropy and Enhanced Dy∙∙∙Dy Coupling in Three Isomers of Dy-Oxide Clusterfullerene Dy2O@C82.Adv Sci (Weinh). 2019 Aug 15;6(20):1901352. doi: 10.1002/advs.201901352. eCollection 2019 Oct 16. Adv Sci (Weinh). 2019. PMID: 31637168 Free PMC article.
References
-
- Heath J. R. et al. Lanthanum complexes of spheroidal carbon shells. J. Am. Chem. Soc. 107, 7779–7780 (1985).
-
- Chai Y. et al. Fullerenes with metals inside. J. Phys. Chem. 95, 7564–7568 (1991).
-
- Akasaka T. & Nagase S. Endofullerenes: a new family of carbon clusters, Kluwer Academic Publishers, Dordrecht ; Boston, 2002.
-
- Yang S. F. & Dunsch L. Endohedral fullerenes. Chapter in “Nanomaterials: Inorganic and Bioinorganic Perspectives” , ed. , John Wiley & Sons, Ltd., Chichester, United Kingdom, 2008, pp 189–214.
-
- Shinohara H. Endohedral metallofullerenes. Rep. Prog. Phys. 63, 843–892 (2000).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

