A novel approach to quantifying ovarian cell lipid content and lipid accumulation in vitro by confocal microscopy in lean women undergoing ovarian stimulation for in vitro fertilization (IVF)
- PMID: 23512091
- PMCID: PMC3663971
- DOI: 10.1007/s10815-013-9976-2
A novel approach to quantifying ovarian cell lipid content and lipid accumulation in vitro by confocal microscopy in lean women undergoing ovarian stimulation for in vitro fertilization (IVF)
Abstract
Purpose: To quantify intracellular lipid levels in cumulus cells (CCs) and mural granulosa cells (MGCs) of lean women undergoing gonadotropin therapy for in vitro fertilization (IVF), based upon different cell preparation methods.
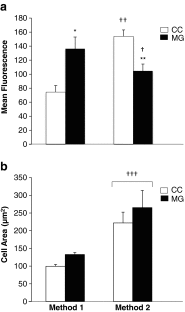
Methods: CCs and MGCs from 16 lean women undergoing ovarian stimulation for IVF were studied. Cells were pooled by cell type, with each type of cell separated into two groups for determination of initial lipid content (Method 1) and subsequent lipid accumulation in vitro (Method 2). Cells for initial lipid content were immediately fixed at the time of the oocyte retrieval with 4% paraformaldehyde in suspension, while those for subsequent lipid accumulation in vitro were cultured for 4 h with 5% fetal calf serum and then fixed. Cells were treated with lipid fluorescent dye BODIPY® FL C16 and nuclear marker DAPI. Intracellular lipid was quantified by confocal microscopy, using ImageJ software analysis.
Results: There was no significant effect of cell type (P = 0.2) or cell type-cell preparation method interaction (P = 0.8) on cell area (Method 1: CC 99.7 ± 5.1, MGC 132.8 ± 5.8; Method 2: CC 221.9 ± 30.4, MGC 265.1 ± 48.5 μm(2)). The mean area of all cells combined was significantly less for cells prepared by Method 1 (116.2 ± 4.9 μm(2)) vs. Method 2 (243.5 ± 22.5 μm(2), P < 0.00005). Intracellular lipid level, however, was significantly altered by cell preparation method (P < 0.05; cell preparation method-cell type interaction, P < 0.00001). Initial lipid content was significantly lower in CC (74.5 ± 9.3) than MGC (136.3 ± 16.7 fluorescence/cell area, P < 0.00005), while subsequent lipid accumulation in vitro was significantly higher in CC (154.0 ± 9.1) than MGC (104.6 ± 9.9 fluorescence/cell area, P < 0.00001). The relatively diminished initial CC lipid content compared to subsequent CC lipid accumulation in vitro (P < 0.00001), and the opposite pattern for MGC (P < 0.05), significantly lowered the CC/MGC lipid ratio in Method 1 (0.55 ± 0.04) vs. Method 2 (1.58 ± 0.10, P < 0.00001).
Conclusions: Differential uptake or utilization of lipid by CC and MGC occurs during oocyte maturation and steroidogenesis, respectively, with the amount of lipid present in ovarian cells a function of both the follicular microenvironment at the time of the oocyte retrieval and the capacity of these cells to accumulate lipid in vitro over time.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

