Cancer cachexia--pathophysiology and management
- PMID: 23512346
- PMCID: PMC3698426
- DOI: 10.1007/s00535-013-0787-0
Cancer cachexia--pathophysiology and management
Abstract
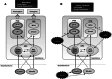
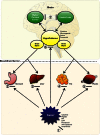
About half of all cancer patients show a syndrome of cachexia, characterized by anorexia and loss of adipose tissue and skeletal muscle mass. Cachexia can have a profound impact on quality of life, symptom burden, and a patient's sense of dignity. It is a very serious complication, as weight loss during cancer treatment is associated with more chemotherapy-related side effects, fewer completed cycles of chemotherapy, and decreased survival rates. Numerous cytokines have been postulated to play a role in the etiology of cancer cachexia. Cytokines can elicit effects that mimic leptin signaling and suppress orexigenic ghrelin and neuropeptide Y (NPY) signaling, inducing sustained anorexia and cachexia not accompanied by the usual compensatory response. Furthermore, cytokines have been implicated in the induction of cancer-related muscle wasting. Cytokine-induced skeletal muscle wasting is probably a multifactorial process, which involves a protein synthesis inhibition, an increase in protein degradation, or a combination of both. The best treatment of the cachectic syndrome is a multifactorial approach. Many drugs including appetite stimulants, thalidomide, cytokine inhibitors, steroids, nonsteroidal anti-inflammatory drugs, branched-chain amino acids, eicosapentaenoic acid, and antiserotoninergic drugs have been proposed and used in clinical trials, while others are still under investigation using experimental animals. There is a growing awareness of the positive impact of supportive care measures and development of promising novel pharmaceutical agents for cachexia. While there has been great progress in understanding the underlying biological mechanisms of cachexia, health care providers must also recognize the psychosocial and biomedical impact cachexia can have.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

