α-Synuclein and mitochondria: partners in crime?
- PMID: 23512373
- PMCID: PMC3701775
- DOI: 10.1007/s13311-013-0182-9
α-Synuclein and mitochondria: partners in crime?
Abstract
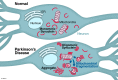
Increased α-synuclein levels and mutations in mitochondria-associated proteins both cause familial Parkinson's disease (PD), and synuclein and mitochondria also play central, but poorly understood, roles in the pathogenesis of idiopathic PD. A fraction of synuclein interacts with mitochondria, and synuclein can produce mitochondrial fragmentation and impair mitochondrial complex I activity. However, the consequences of these mitochondrial changes for bioenergetic and other mitochondrial functions remain poorly defined, as does the role of synuclein-mitochondria interactions in the normal and pathologic effects of synuclein. Understanding the functional consequences of synuclein's interactions with mitochondria is likely to provide important insights into disease pathophysiology, and may also reveal therapeutic strategies.
Figures

References
-
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl. 5):S21–S23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

